| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1292330 | 1497926 | 2016 | 7 صفحه PDF | دانلود رایگان |
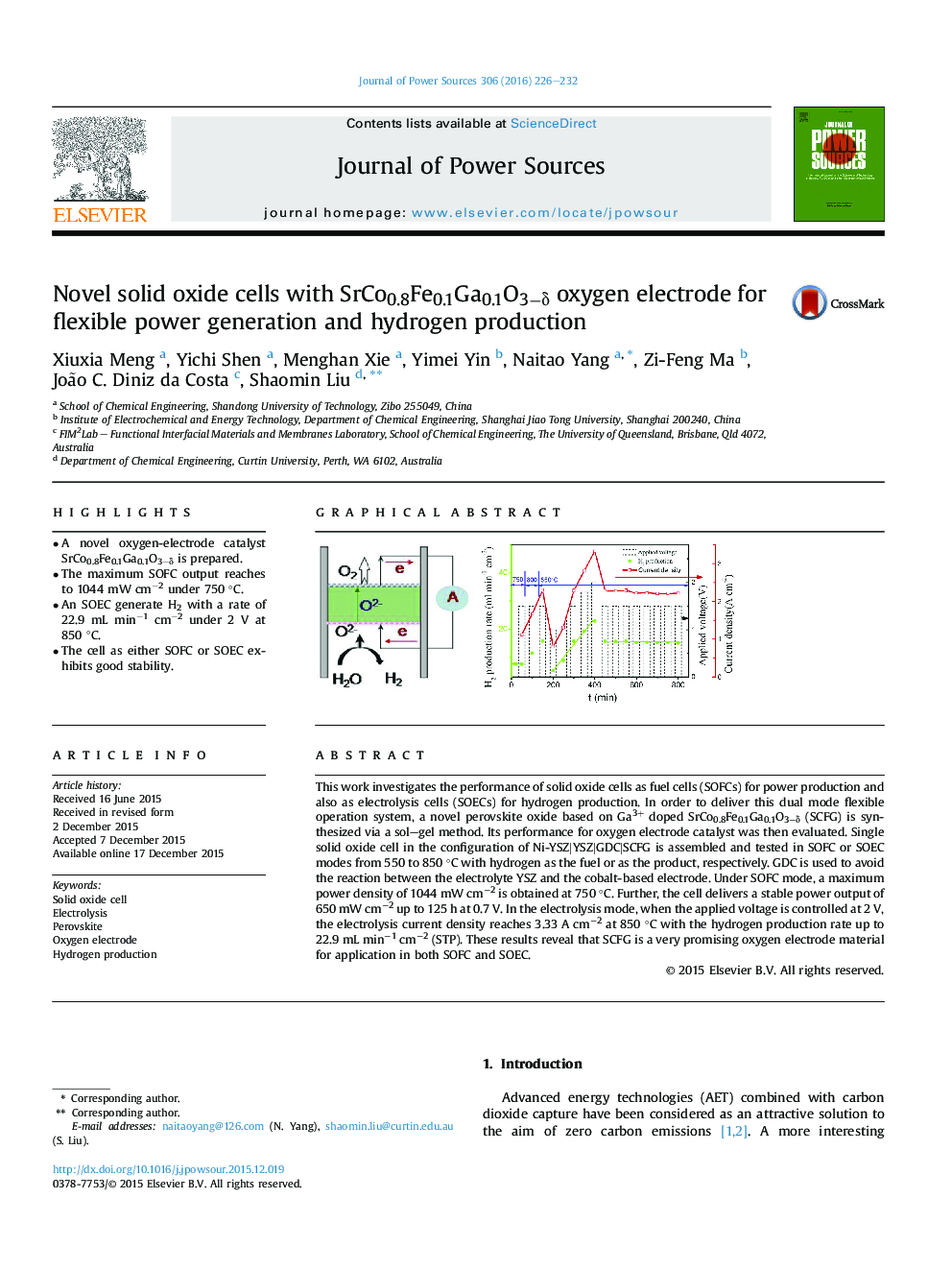
• A novel oxygen-electrode catalyst SrCo0.8Fe0.1Ga0.1O3−δ is prepared.
• The maximum SOFC output reaches to 1044 mW cm−2 under 750 °C.
• An SOEC generate H2 with a rate of 22.9 mL min−1 cm−2 under 2 V at 850 °C.
• The cell as either SOFC or SOEC exhibits good stability.
This work investigates the performance of solid oxide cells as fuel cells (SOFCs) for power production and also as electrolysis cells (SOECs) for hydrogen production. In order to deliver this dual mode flexible operation system, a novel perovskite oxide based on Ga3+ doped SrCo0.8Fe0.1Ga0.1O3−δ (SCFG) is synthesized via a sol–gel method. Its performance for oxygen electrode catalyst was then evaluated. Single solid oxide cell in the configuration of Ni-YSZ|YSZ|GDC|SCFG is assembled and tested in SOFC or SOEC modes from 550 to 850 °C with hydrogen as the fuel or as the product, respectively. GDC is used to avoid the reaction between the electrolyte YSZ and the cobalt-based electrode. Under SOFC mode, a maximum power density of 1044 mW cm−2 is obtained at 750 °C. Further, the cell delivers a stable power output of 650 mW cm−2 up to 125 h at 0.7 V. In the electrolysis mode, when the applied voltage is controlled at 2 V, the electrolysis current density reaches 3.33 A cm−2 at 850 °C with the hydrogen production rate up to 22.9 mL min−1 cm−2 (STP). These results reveal that SCFG is a very promising oxygen electrode material for application in both SOFC and SOEC.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 306, 29 February 2016, Pages 226–232