| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1312014 | 1499145 | 2016 | 9 صفحه PDF | دانلود رایگان |
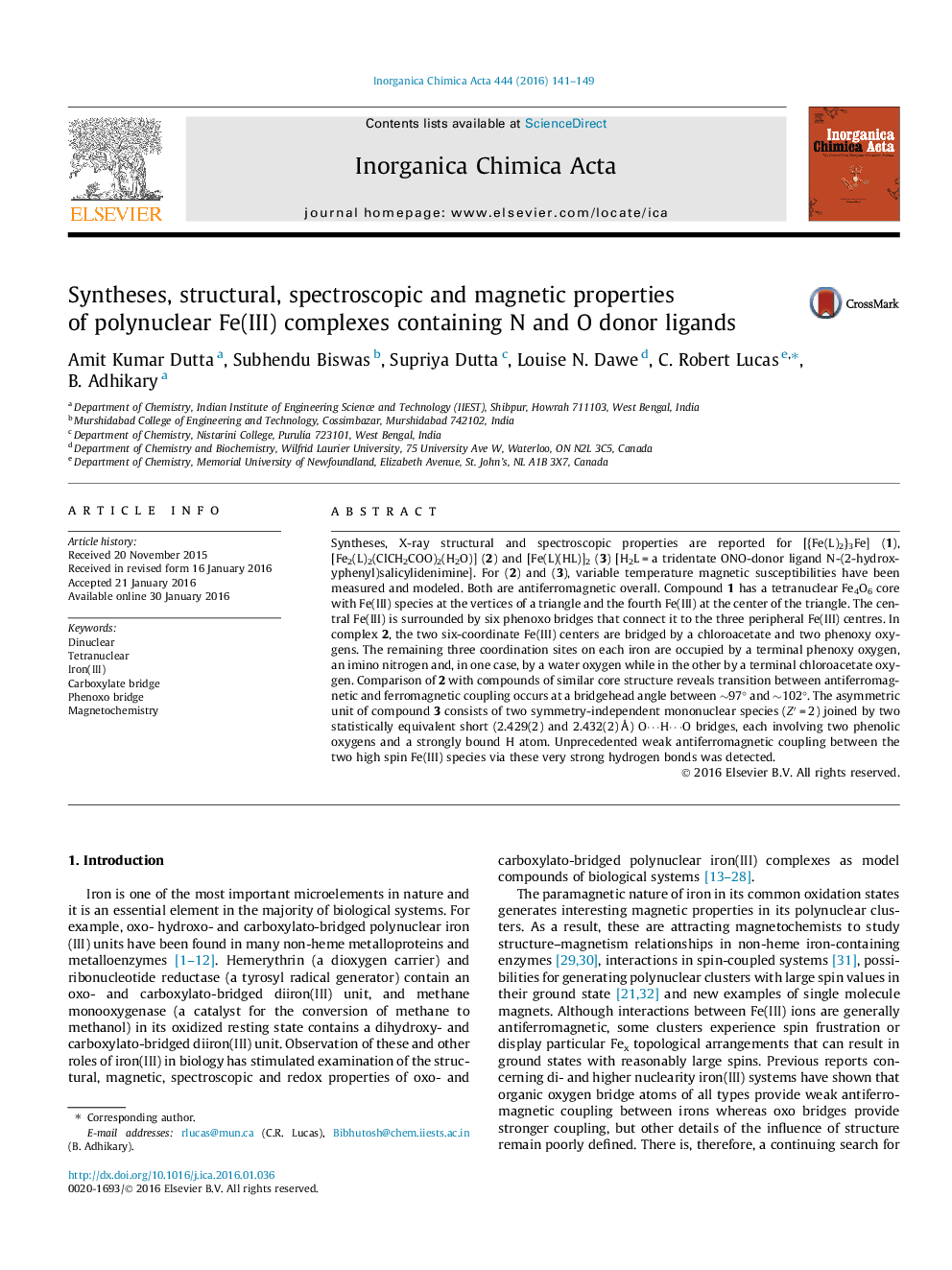
• [{Fe(L)2}3Fe] 1, [Fe2(L)2(ClCH2COO)2(H2O)] 2 and [Fe(L)(HL)]23 are synthesized.
• Intramolecular superexchange in 2 and 3 is small and antiferromagnetic.
• Cores like 2 go from antiferro- to ferromagnetic at bridge angles between ∼97° and ∼102°.
• 3 is a dimer held together by two very strong hydrogen bonds.
• Antiferromagnetic coupling in 3 occurs via the hydrogen bonds.
Syntheses, X-ray structural and spectroscopic properties are reported for [{Fe(L)2}3Fe] (1), [Fe2(L)2(ClCH2COO)2(H2O)] (2) and [Fe(L)(HL)]2 (3) [H2L = a tridentate ONO-donor ligand N-(2-hydroxyphenyl)salicylidenimine]. For (2) and (3), variable temperature magnetic susceptibilities have been measured and modeled. Both are antiferromagnetic overall. Compound 1 has a tetranuclear Fe4O6 core with Fe(III) species at the vertices of a triangle and the fourth Fe(III) at the center of the triangle. The central Fe(III) is surrounded by six phenoxo bridges that connect it to the three peripheral Fe(III) centres. In complex 2, the two six-coordinate Fe(III) centers are bridged by a chloroacetate and two phenoxy oxygens. The remaining three coordination sites on each iron are occupied by a terminal phenoxy oxygen, an imino nitrogen and, in one case, by a water oxygen while in the other by a terminal chloroacetate oxygen. Comparison of 2 with compounds of similar core structure reveals transition between antiferromagnetic and ferromagnetic coupling occurs at a bridgehead angle between ∼97° and ∼102°. The asymmetric unit of compound 3 consists of two symmetry-independent mononuclear species (Z′ = 2) joined by two statistically equivalent short (2.429(2) and 2.432(2) Å) O⋯H⋯O bridges, each involving two phenolic oxygens and a strongly bound H atom. Unprecedented weak antiferromagnetic coupling between the two high spin Fe(III) species via these very strong hydrogen bonds was detected.
Magnetostructural properties of two compounds are reported. From one, a range of bridgehead angles within which coupling changes from ferromagnetic to antiferromagnetic is established. From the other (below), antiferromagnetic coupling between high spin Fe(III) centres via two very strong hydrogen bonds is observed.Figure optionsDownload as PowerPoint slide
Journal: Inorganica Chimica Acta - Volume 444, 1 April 2016, Pages 141–149