| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1321535 | 1499894 | 2013 | 9 صفحه PDF | دانلود رایگان |
• Synthesis of glycosylated heterobimetallic CuII/NiII–Sn2IV chemical entities 1–4.
• In vitro DNA binding studies revealed electrostatic interactions and hydrogen bonding.
• Oxidative cleavage pathway was suggested for complex 3.
• Molecular docking studies were consistent with the binding of 3 in minor groove of DNA.
• 3 Exhibited good cytotoxic activity against leukemia (K-562) cell line.
Carbohydrate-conjugated heterobimetallic CuII/NiII–Sn2IV chemical entities 1–4 were designed, synthesized and characterized by elemental analysis and spectroscopic methods viz., UV–vis, IR, ESI–mass and NMR (1H, 13C, and 119Sn; in case of NiII–Sn2IV analogs 2 and 4) and EPR (in case of CuII–Sn2IV analogs 1 and 3) to act as specific antitumor chemotherapeutics. Interaction of 1 and 3 with CT DNA was carried out by optical methods to ascertain the mode of binding, mechanism of action and their therapeutic potential. 1 and 3 bind to DNA via electrostatic mode involving phosphate oxygen of DNA-sugar backbone. The intrinsic binding constant, Kb values of 1 and 3, were calculated and found to be 2.76 × 104 and 3.84 × 104 M−1, respectively which implicates greater binding propensity of diphenyltin analog 3. Furthermore, a mechanistic insight with 5′-GMP was envisaged for 1 and 3. The cleavage activity of 3 was investigated with supercoiled pBR322 DNA by gel electrophoresis which demonstrated efficient cleavage activity via an oxidative pathway. The molecular docking technique was also utilized to ascertain the mechanism and mode of action of diphenyltin analog 3. The in vitro antitumor activity of 3 was evaluated by SRB assay against a panel of human cancer cell lines which clearly revealed good cytotoxic activity against K-562 human leukemic cells with GI50 values <10 μg/ml.
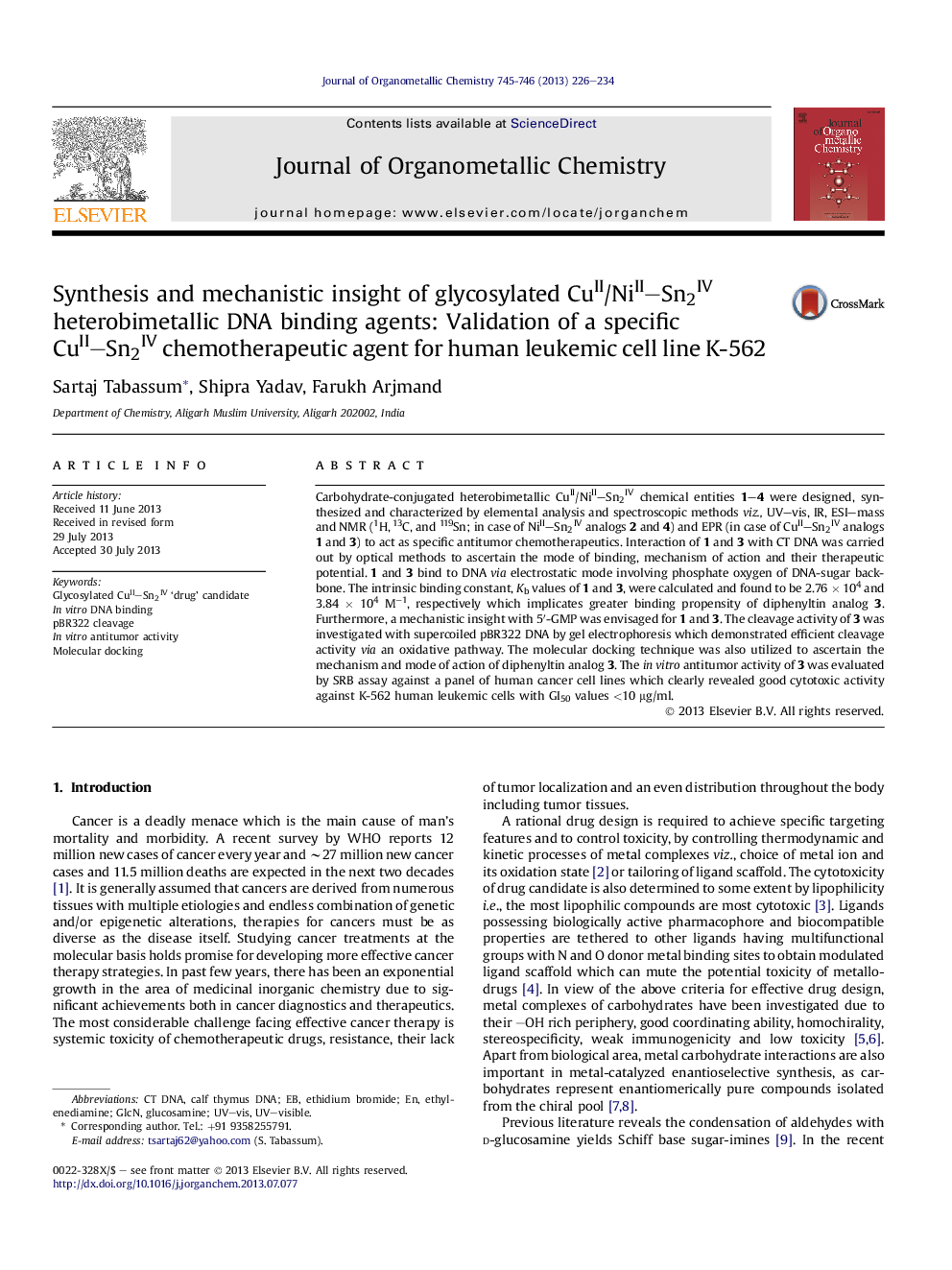
The predicted top-ranking lowest energy molecular docked pose of chemotherapeutic agent 3 with the DNA.Figure optionsDownload as PowerPoint slide
Journal: Journal of Organometallic Chemistry - Volumes 745–746, 15 November 2013, Pages 226–234