| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1325352 | 977379 | 2011 | 4 صفحه PDF | دانلود رایگان |
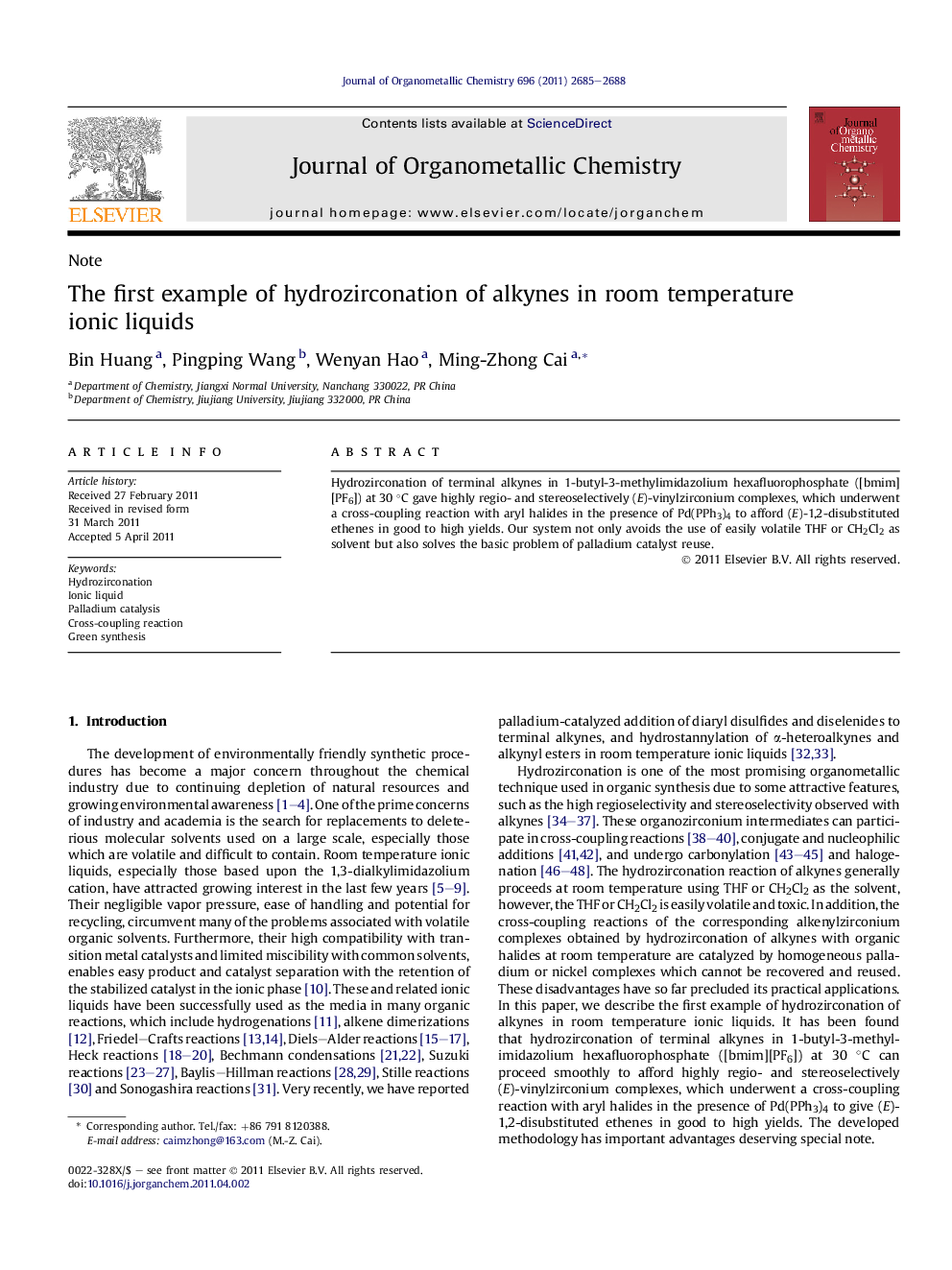
Hydrozirconation of terminal alkynes in 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]) at 30 °C gave highly regio- and stereoselectively (E)-vinylzirconium complexes, which underwent a cross-coupling reaction with aryl halides in the presence of Pd(PPh3)4 to afford (E)-1,2-disubstituted ethenes in good to high yields. Our system not only avoids the use of easily volatile THF or CH2Cl2 as solvent but also solves the basic problem of palladium catalyst reuse.
Hydrozirconation of terminal alkynes in [bmim][PF6] at 30 °C gave highly regio- and stereoselectively (E)-vinylzirconium complexes, which underwent a palladium- catalyzed cross-coupling reaction with aryl halides to afford (E)-1,2-disubstituted ethenes in good to high yields. The ionic liquid and palladium catalyst can be recycled at least six times.Figure optionsDownload as PowerPoint slideHighlights
► Hydrozirconation of alkynes in ionic liquids was firstly investigated.
► The reaction proceeded highly regio- and stereoselectively in [bmim][PF6].
► The coupling of (E)-vinylzirconium complexes with aryl halides was also achieved.
► The ionic liquid and palladium catalyst can be recycled at least six times.
Journal: Journal of Organometallic Chemistry - Volume 696, Issue 13, 1 July 2011, Pages 2685–2688