| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1355222 | 980827 | 2011 | 8 صفحه PDF | دانلود رایگان |
Methylxanthines (MTX), in particular caffeine (CAF), are known as the most widely consumed alkaloids worldwide. Many accumulated statistical data indicate the protective effect of CAF intake against several types of cancer. One of the possible explanations of this phenomenon is direct non-covalent interaction between CAF and aromatic mutagen/carcinogen molecules through stacking (π–π) complexes formation. Here we demonstrate that CAF and other MTX, pentoxifylline (PTX) and theophylline (TH), form stacking complexes with carcinogenic imidazoquinoline-type (IQ-type) food-borne heterocyclic aromatic amines (HCAs). We estimated neighborhood association constants (KAC of the order of magnitude of 102 M−1) in neutral and acidic environment and enthalpy changes (ΔH values between −15.1 and −39.8 kJ/mol) for these interactions using UV–Vis spectroscopy, calculations based on thermodynamical model of mixed aggregation and titration microcalorimetry. Moreover, using Ames test with Salmonella typhimurium TA98 strain and recently developed mutagenicity assay based on bioluminescence of Vibrio harveyi A16 strain, we demonstrated a statistically significant reduction in HCAs mutagenic activity in the presence of MTX.
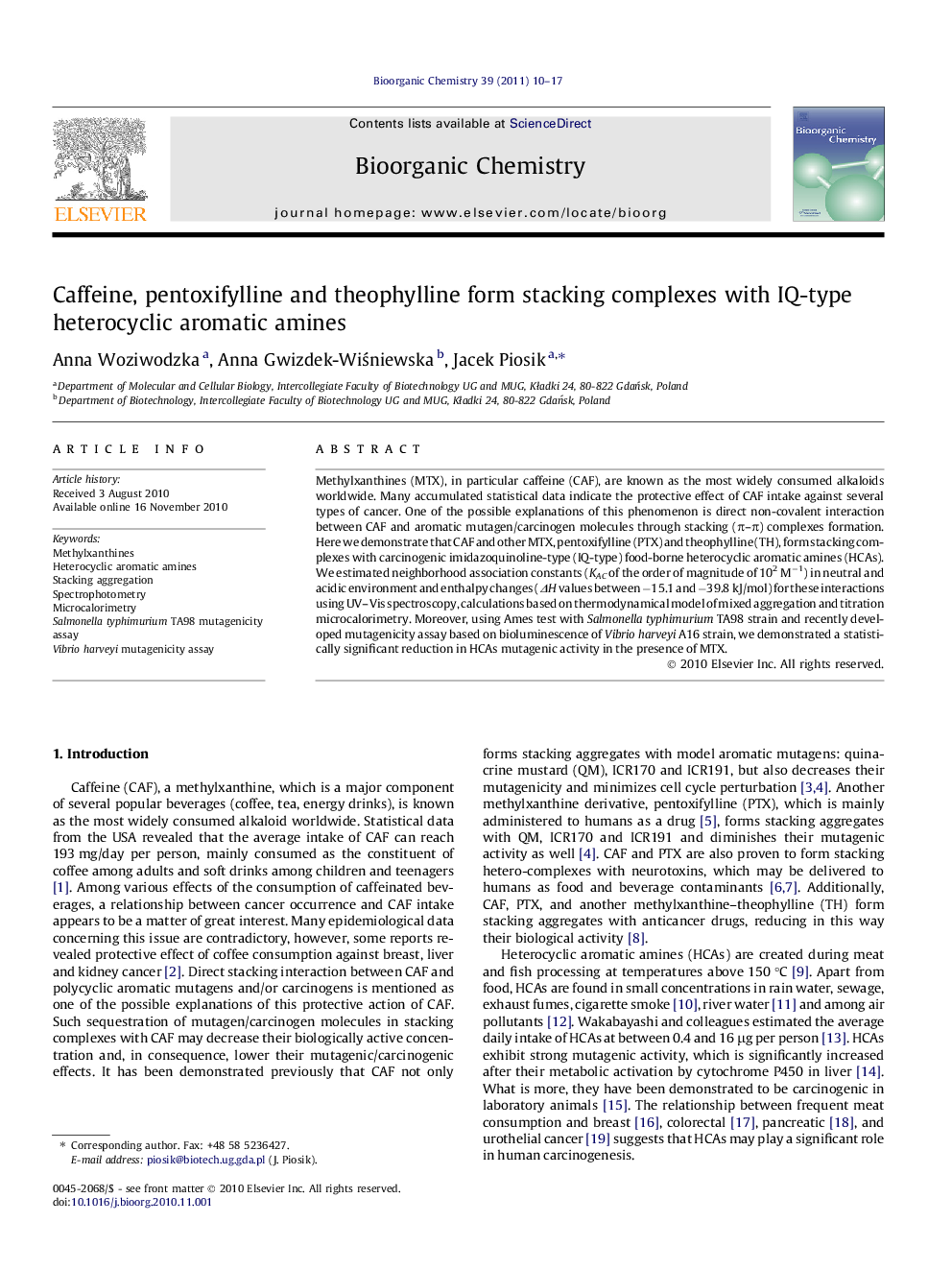
Visualization of the most probable lowest energy stacking complex between Caffeine and 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) from the top view (left panel) and from the side view (right panel), obtained by molecular modeling.Figure optionsDownload as PowerPoint slideResearch highlights
► Caffeine and other methylxanthines form stacking complexes with IQ-type HCAs.
► Calculated association constants are in the order of magnitude about 102 M−1.
► Enthalpy changes ΔH values were estimated between −15.1 and −39.8 kJ/mol.
► Mixed complex formation leads to the decrease in HCAs mutagenicity.
Journal: Bioorganic Chemistry - Volume 39, Issue 1, February 2011, Pages 10–17