| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1355805 | 981065 | 2012 | 10 صفحه PDF | دانلود رایگان |
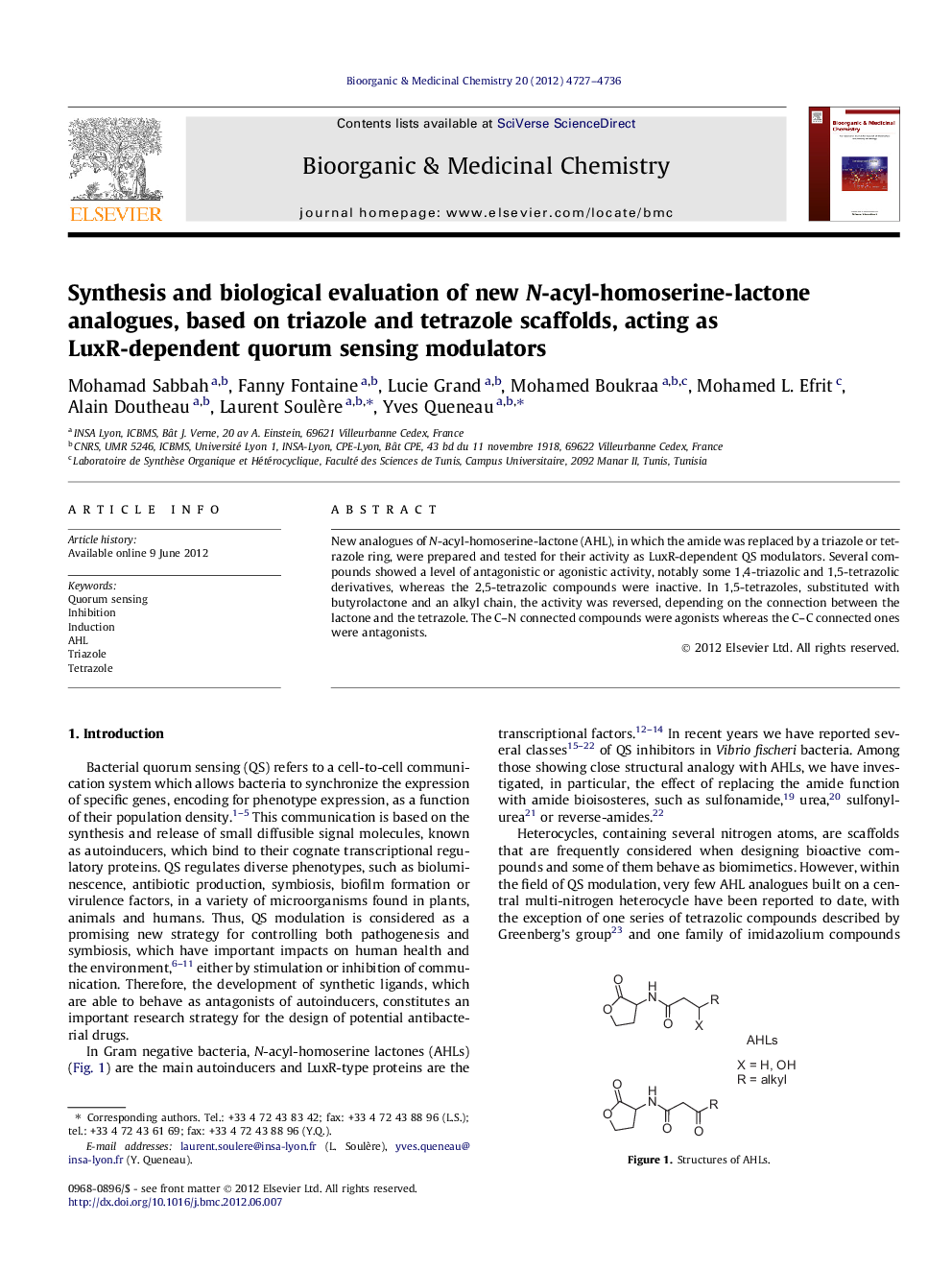
New analogues of N-acyl-homoserine-lactone (AHL), in which the amide was replaced by a triazole or tetrazole ring, were prepared and tested for their activity as LuxR-dependent QS modulators. Several compounds showed a level of antagonistic or agonistic activity, notably some 1,4-triazolic and 1,5-tetrazolic derivatives, whereas the 2,5-tetrazolic compounds were inactive. In 1,5-tetrazoles, substituted with butyrolactone and an alkyl chain, the activity was reversed, depending on the connection between the lactone and the tetrazole. The C–N connected compounds were agonists whereas the C–C connected ones were antagonists.
New 1,2,3-triazolic or 1,2,3,4-tetrazolic N-acyl-homoserine-lactone analogues showed activities either antagonist or agonist in LuxR dependent quorum sensing, notably some 1,4-triazolic and 1,5-tetrazolic ones, whereas 2,5-tetrazolic systems are inactive. Activity (agonistic or antagonistic) also depends on the connection between the heterocycle and the lactone moiety.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 20, Issue 15, 1 August 2012, Pages 4727–4736