| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1355991 | 981080 | 2011 | 4 صفحه PDF | دانلود رایگان |
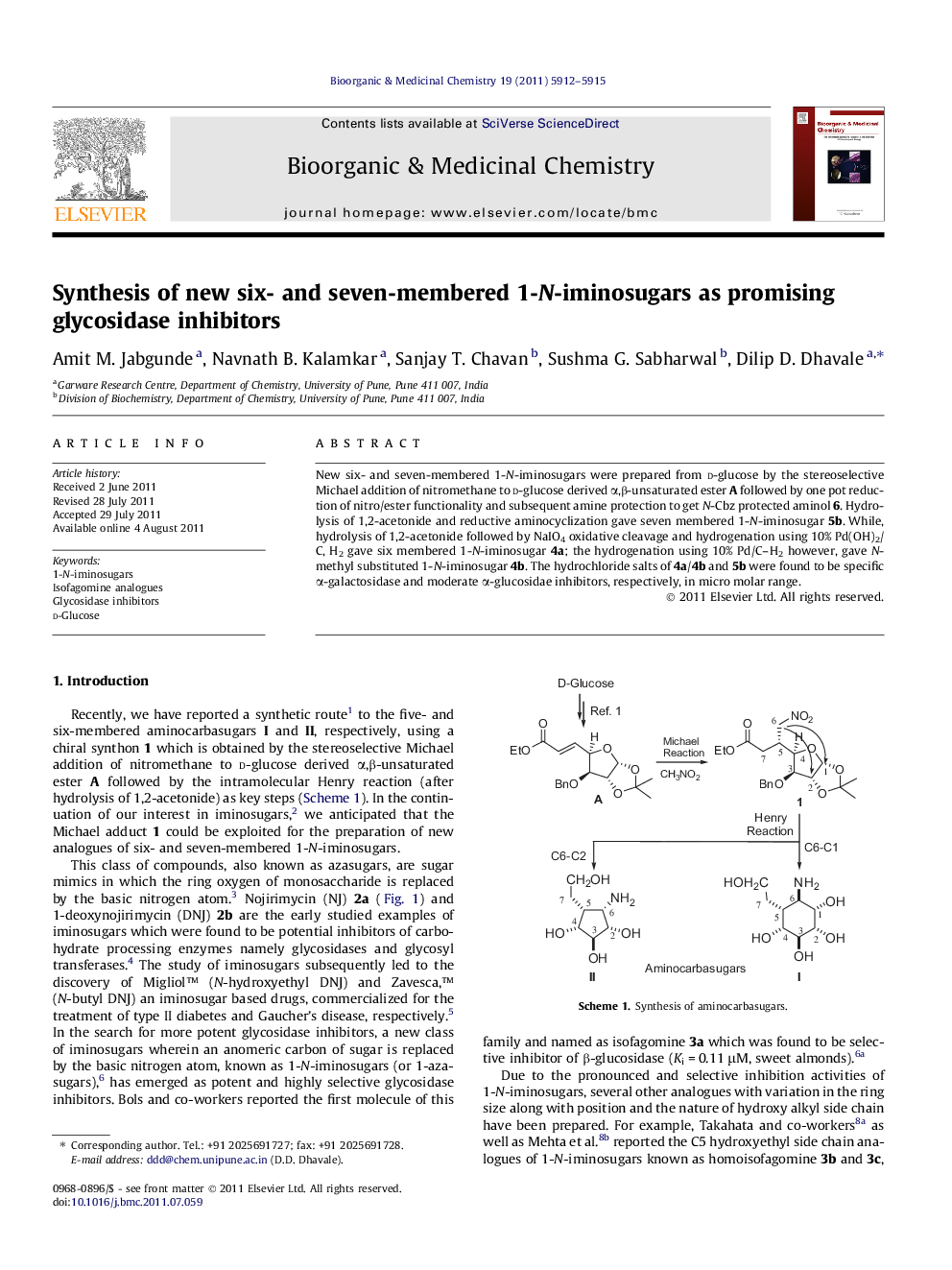
New six- and seven-membered 1-N-iminosugars were prepared from d-glucose by the stereoselective Michael addition of nitromethane to d-glucose derived α,β-unsaturated ester A followed by one pot reduction of nitro/ester functionality and subsequent amine protection to get N-Cbz protected aminol 6. Hydrolysis of 1,2-acetonide and reductive aminocyclization gave seven membered 1-N-iminosugar 5b. While, hydrolysis of 1,2-acetonide followed by NaIO4 oxidative cleavage and hydrogenation using 10% Pd(OH)2/C, H2 gave six membered 1-N-iminosugar 4a; the hydrogenation using 10% Pd/C–H2 however, gave N-methyl substituted 1-N-iminosugar 4b. The hydrochloride salts of 4a/4b and 5b were found to be specific α-galactosidase and moderate α-glucosidae inhibitors, respectively, in micro molar range.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 19, Issue 19, 1 October 2011, Pages 5912–5915