| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1358631 | 981351 | 2012 | 9 صفحه PDF | دانلود رایگان |
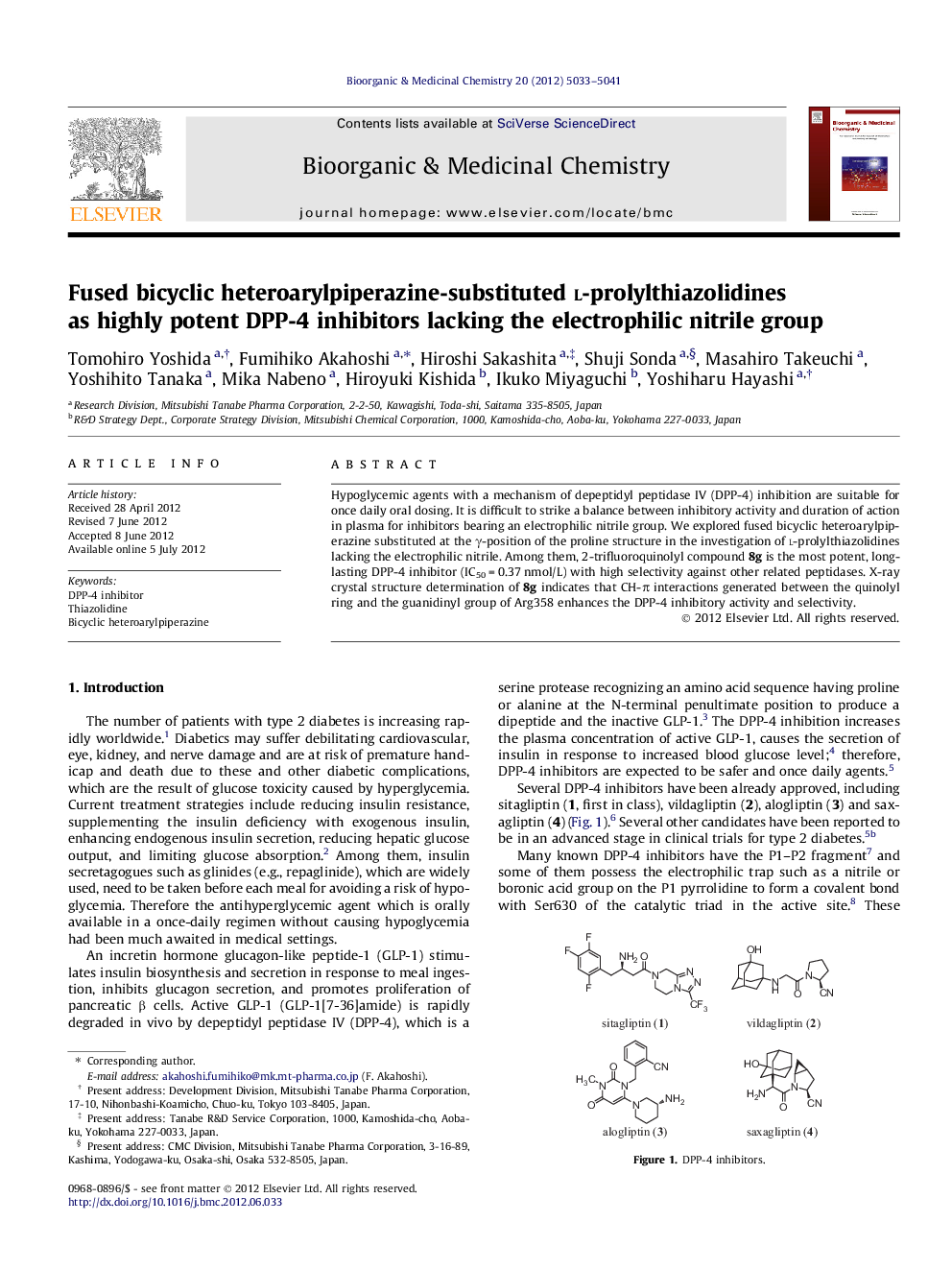
Hypoglycemic agents with a mechanism of depeptidyl peptidase IV (DPP-4) inhibition are suitable for once daily oral dosing. It is difficult to strike a balance between inhibitory activity and duration of action in plasma for inhibitors bearing an electrophilic nitrile group. We explored fused bicyclic heteroarylpiperazine substituted at the γ-position of the proline structure in the investigation of l-prolylthiazolidines lacking the electrophilic nitrile. Among them, 2-trifluoroquinolyl compound 8g is the most potent, long-lasting DPP-4 inhibitor (IC50 = 0.37 nmol/L) with high selectivity against other related peptidases. X-ray crystal structure determination of 8g indicates that CH-π interactions generated between the quinolyl ring and the guanidinyl group of Arg358 enhances the DPP-4 inhibitory activity and selectivity.
A series of prolylthiazolidines with fused bicyclic heteroarylpiperazine substitution has been discovered as superior DPP-4 inhibitors, and the most potent 8g showed high selectivity and in vivo efficacy.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 20, Issue 16, 15 August 2012, Pages 5033–5041