| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1358942 | 981374 | 2011 | 5 صفحه PDF | دانلود رایگان |
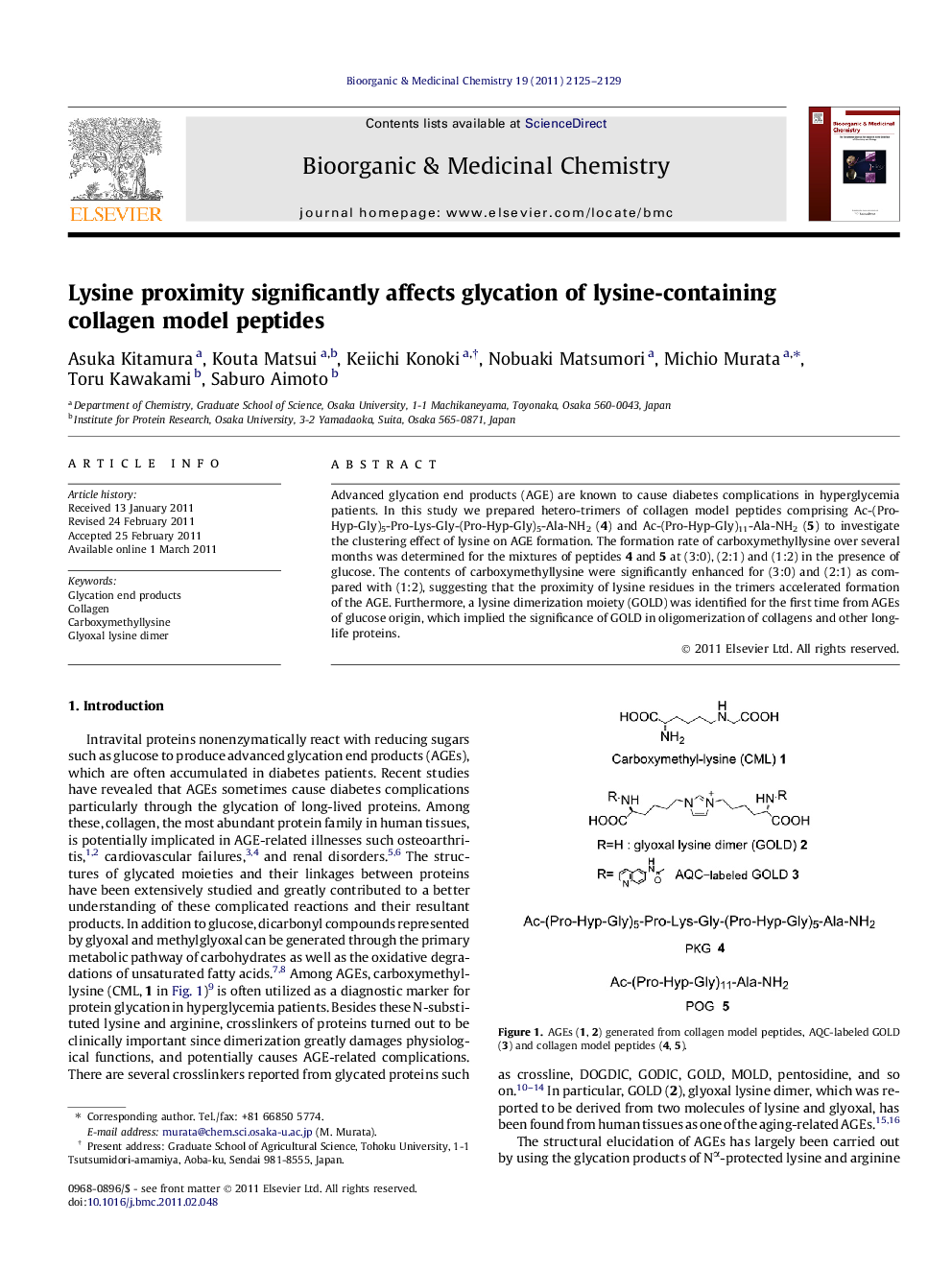
Advanced glycation end products (AGE) are known to cause diabetes complications in hyperglycemia patients. In this study we prepared hetero-trimers of collagen model peptides comprising Ac-(Pro-Hyp-Gly)5-Pro-Lys-Gly-(Pro-Hyp-Gly)5-Ala-NH2 (4) and Ac-(Pro-Hyp-Gly)11-Ala-NH2 (5) to investigate the clustering effect of lysine on AGE formation. The formation rate of carboxymethyllysine over several months was determined for the mixtures of peptides 4 and 5 at (3:0), (2:1) and (1:2) in the presence of glucose. The contents of carboxymethyllysine were significantly enhanced for (3:0) and (2:1) as compared with (1:2), suggesting that the proximity of lysine residues in the trimers accelerated formation of the AGE. Furthermore, a lysine dimerization moiety (GOLD) was identified for the first time from AGEs of glucose origin, which implied the significance of GOLD in oligomerization of collagens and other long-life proteins.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 19, Issue 7, 1 April 2011, Pages 2125–2129