| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1359241 | 981398 | 2010 | 11 صفحه PDF | دانلود رایگان |
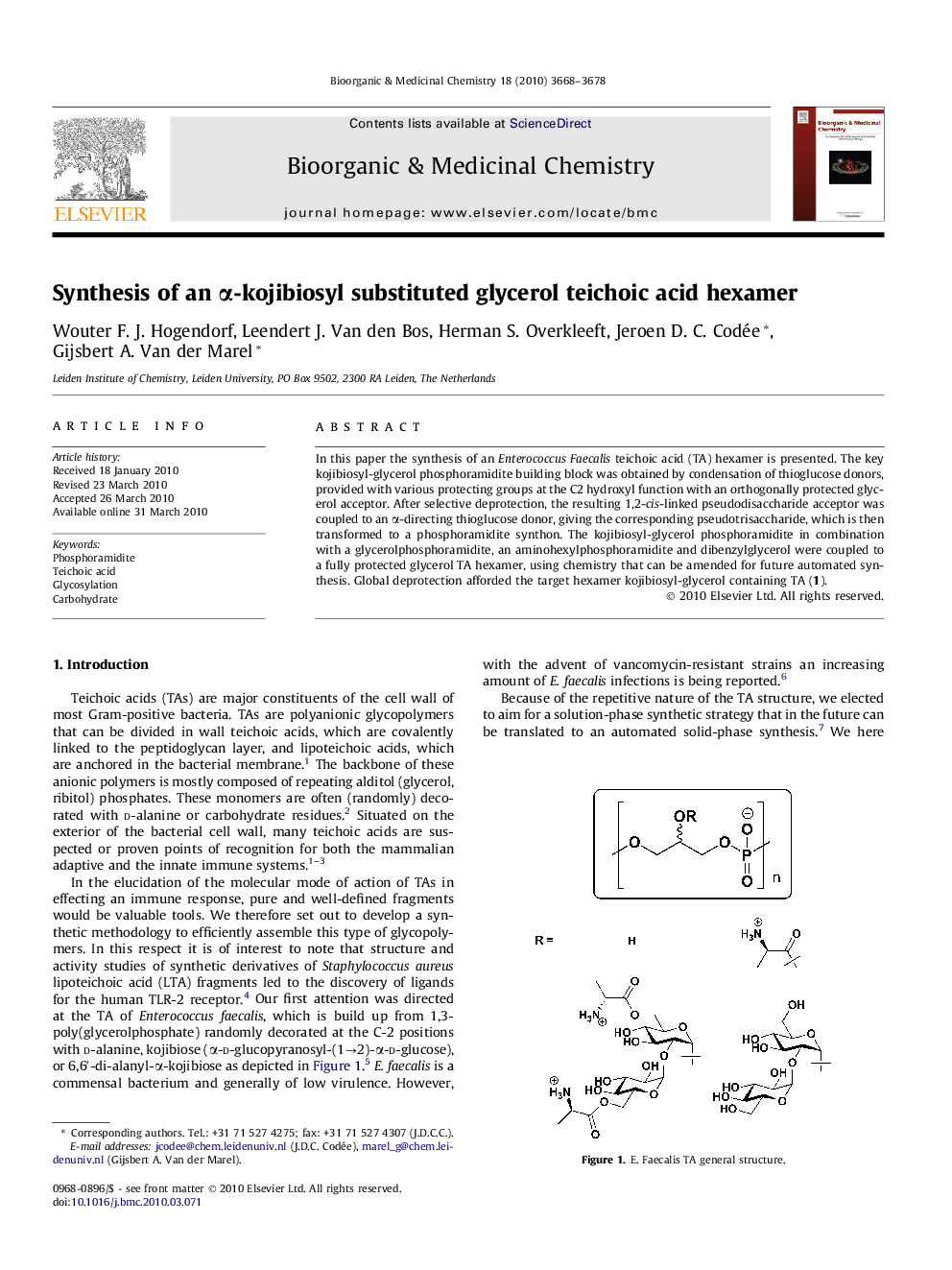
In this paper the synthesis of an Enterococcus Faecalis teichoic acid (TA) hexamer is presented. The key kojibiosyl-glycerol phosphoramidite building block was obtained by condensation of thioglucose donors, provided with various protecting groups at the C2 hydroxyl function with an orthogonally protected glycerol acceptor. After selective deprotection, the resulting 1,2-cis-linked pseudodisaccharide acceptor was coupled to an α-directing thioglucose donor, giving the corresponding pseudotrisaccharide, which is then transformed to a phosphoramidite synthon. The kojibiosyl-glycerol phosphoramidite in combination with a glycerolphosphoramidite, an aminohexylphosphoramidite and dibenzylglycerol were coupled to a fully protected glycerol TA hexamer, using chemistry that can be amended for future automated synthesis. Global deprotection afforded the target hexamer kojibiosyl-glycerol containing TA (1).
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 18, Issue 11, 1 June 2010, Pages 3668–3678