| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1360376 | 981434 | 2008 | 11 صفحه PDF | دانلود رایگان |
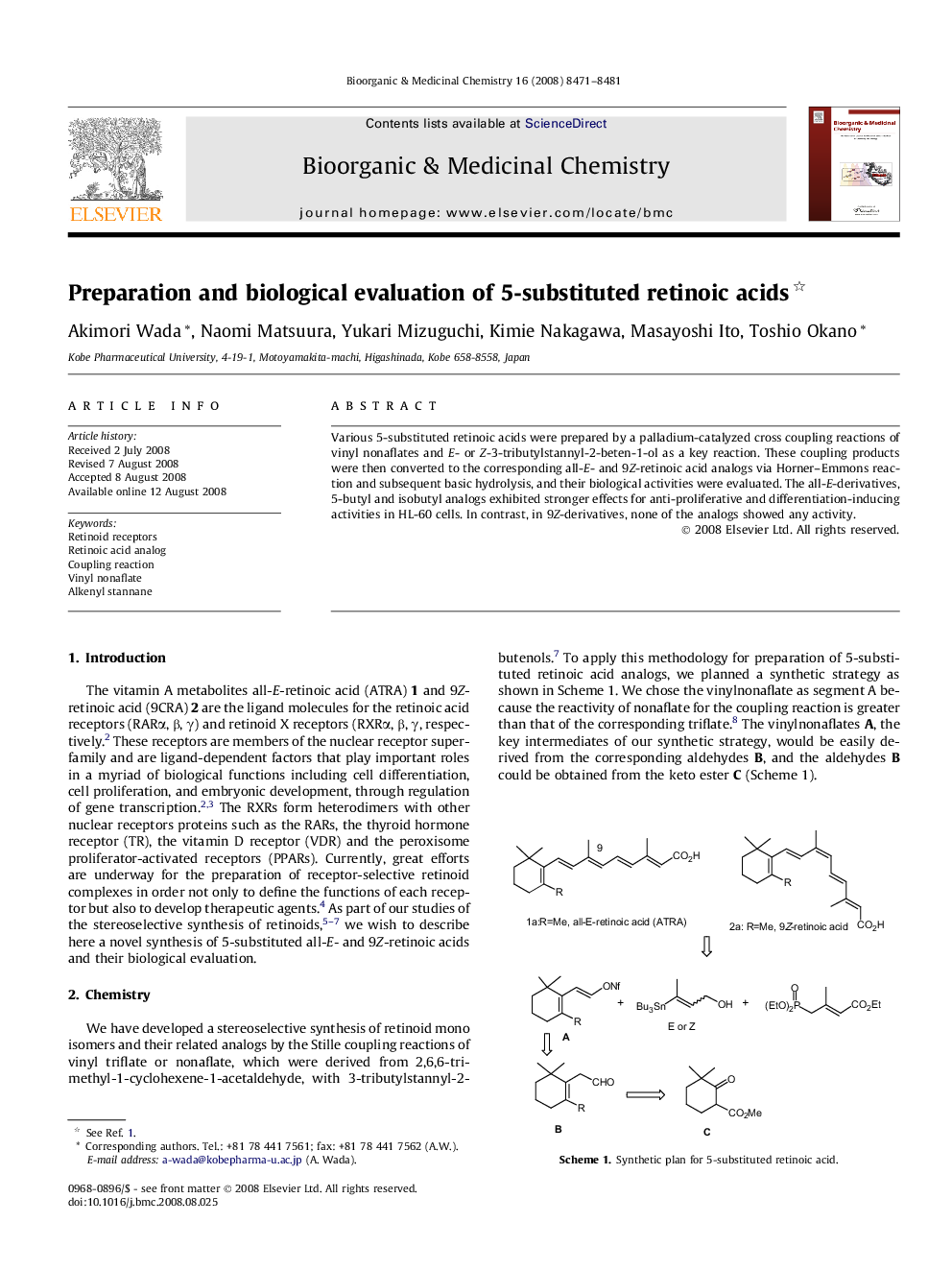
Various 5-substituted retinoic acids were prepared by a palladium-catalyzed cross coupling reactions of vinyl nonaflates and E- or Z-3-tributylstannyl-2-beten-1-ol as a key reaction. These coupling products were then converted to the corresponding all-E- and 9Z-retinoic acid analogs via Horner–Emmons reaction and subsequent basic hydrolysis, and their biological activities were evaluated. The all-E-derivatives, 5-butyl and isobutyl analogs exhibited stronger effects for anti-proliferative and differentiation-inducing activities in HL-60 cells. In contrast, in 9Z-derivatives, none of the analogs showed any activity.
A novel method for a synthesis of 5-substituted retinoic acids analogs was developed by a palladium catalyzed cross-coupling reaction of enol nonaflate with alkenylstannane. The biological activities of these analogs were evaluated.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 16, Issue 18, 15 September 2008, Pages 8471–8481