| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1361551 | 981466 | 2008 | 6 صفحه PDF | دانلود رایگان |
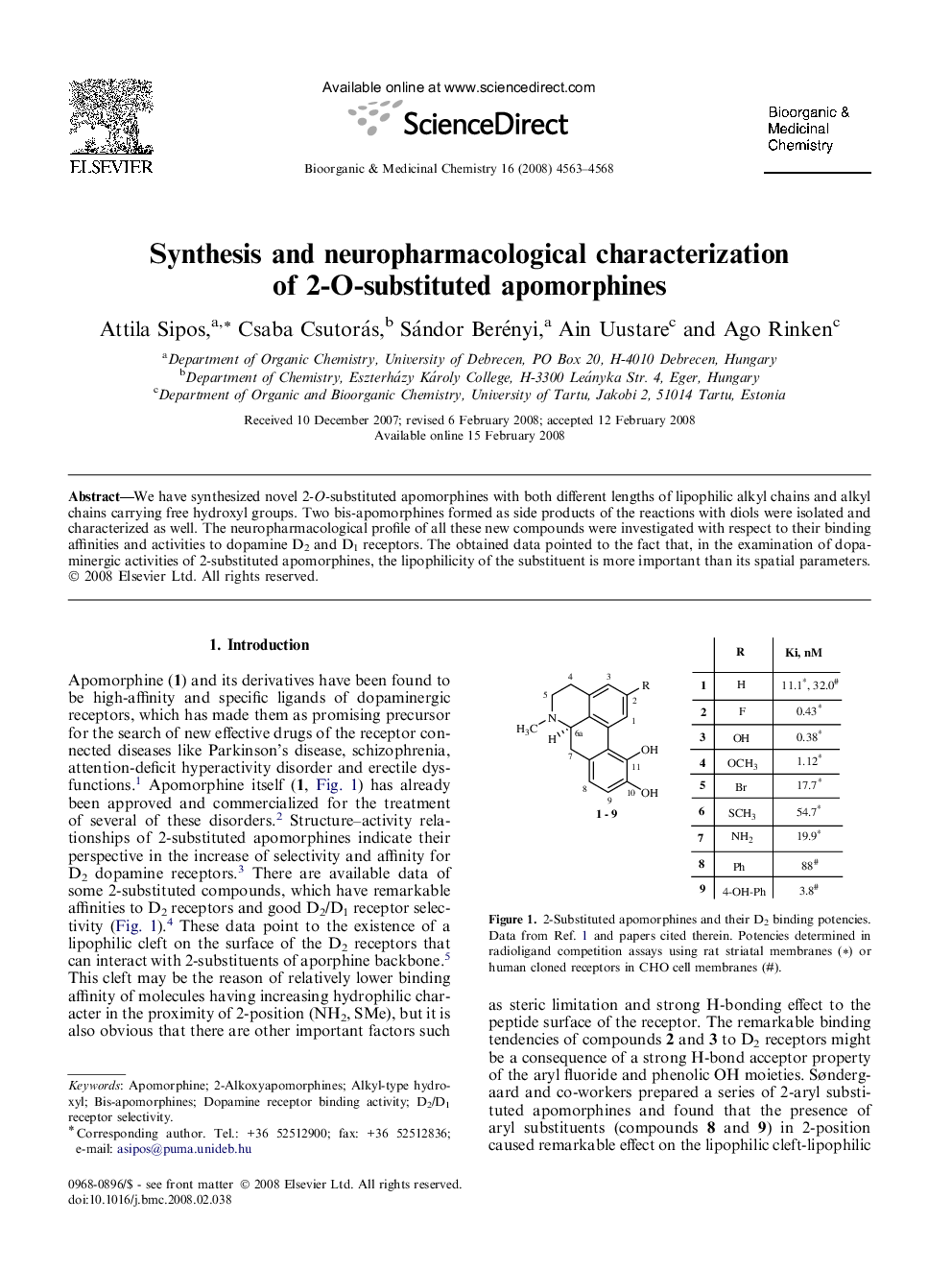
We have synthesized novel 2-O-substituted apomorphines with both different lengths of lipophilic alkyl chains and alkyl chains carrying free hydroxyl groups. Two bis-apomorphines formed as side products of the reactions with diols were isolated and characterized as well. The neuropharmacological profile of all these new compounds were investigated with respect to their binding affinities and activities to dopamine D2 and D1 receptors. The obtained data pointed to the fact that, in the examination of dopaminergic activities of 2-substituted apomorphines, the lipophilicity of the substituent is more important than its spatial parameters.
We have synthesized novel 2-O-substituted apomorphines with both different lengths of lipophilic alkyl chains and alkyl chains carrying free hydroxyl groups. Two bis-apomorphines formed as side products of the reactions with diols were isolated and characterized as well. The neuropharmacological profile of all these new compounds was investigated with respect to their binding affinities and activities to dopamine D2 and D1 receptors. The obtained data pointed to the fact that, in the examination of dopaminergic activities of 2-substituted apomorphines, the lipophilicity of the substituent is more important than its spatial parameters.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 16, Issue 8, 15 April 2008, Pages 4563–4568