| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1362153 | 981479 | 2010 | 16 صفحه PDF | دانلود رایگان |
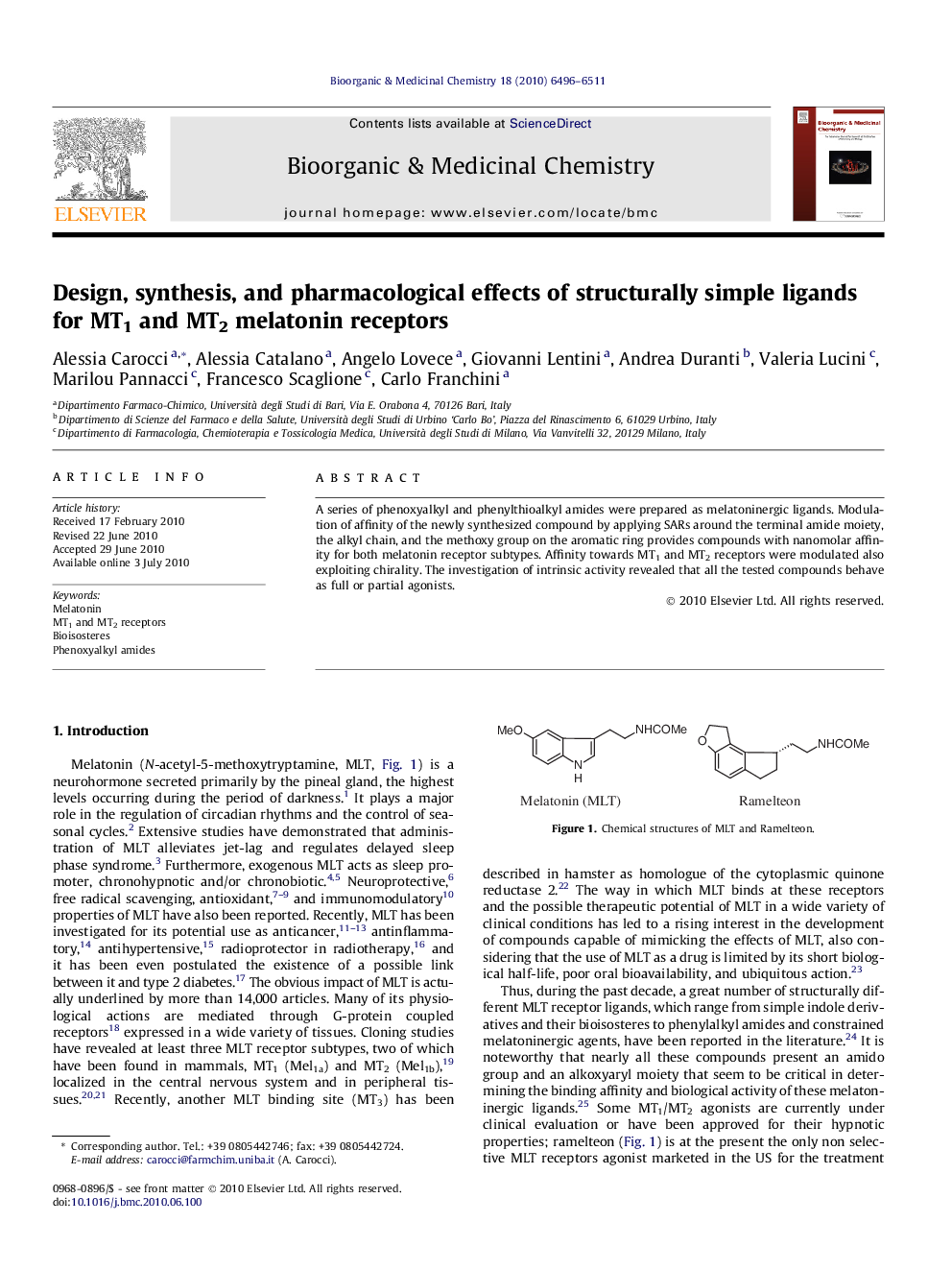
A series of phenoxyalkyl and phenylthioalkyl amides were prepared as melatoninergic ligands. Modulation of affinity of the newly synthesized compound by applying SARs around the terminal amide moiety, the alkyl chain, and the methoxy group on the aromatic ring provides compounds with nanomolar affinity for both melatonin receptor subtypes. Affinity towards MT1 and MT2 receptors were modulated also exploiting chirality. The investigation of intrinsic activity revealed that all the tested compounds behave as full or partial agonists.
A novel series of phenoxyalkyl and phenylthioalkyl amides were synthesized and their structure–affinity and structure–intrinsic activity relationships for MT1 and MT2 receptors were investigated.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 18, Issue 17, 1 September 2010, Pages 6496–6511