| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1362605 | 981492 | 2006 | 8 صفحه PDF | دانلود رایگان |
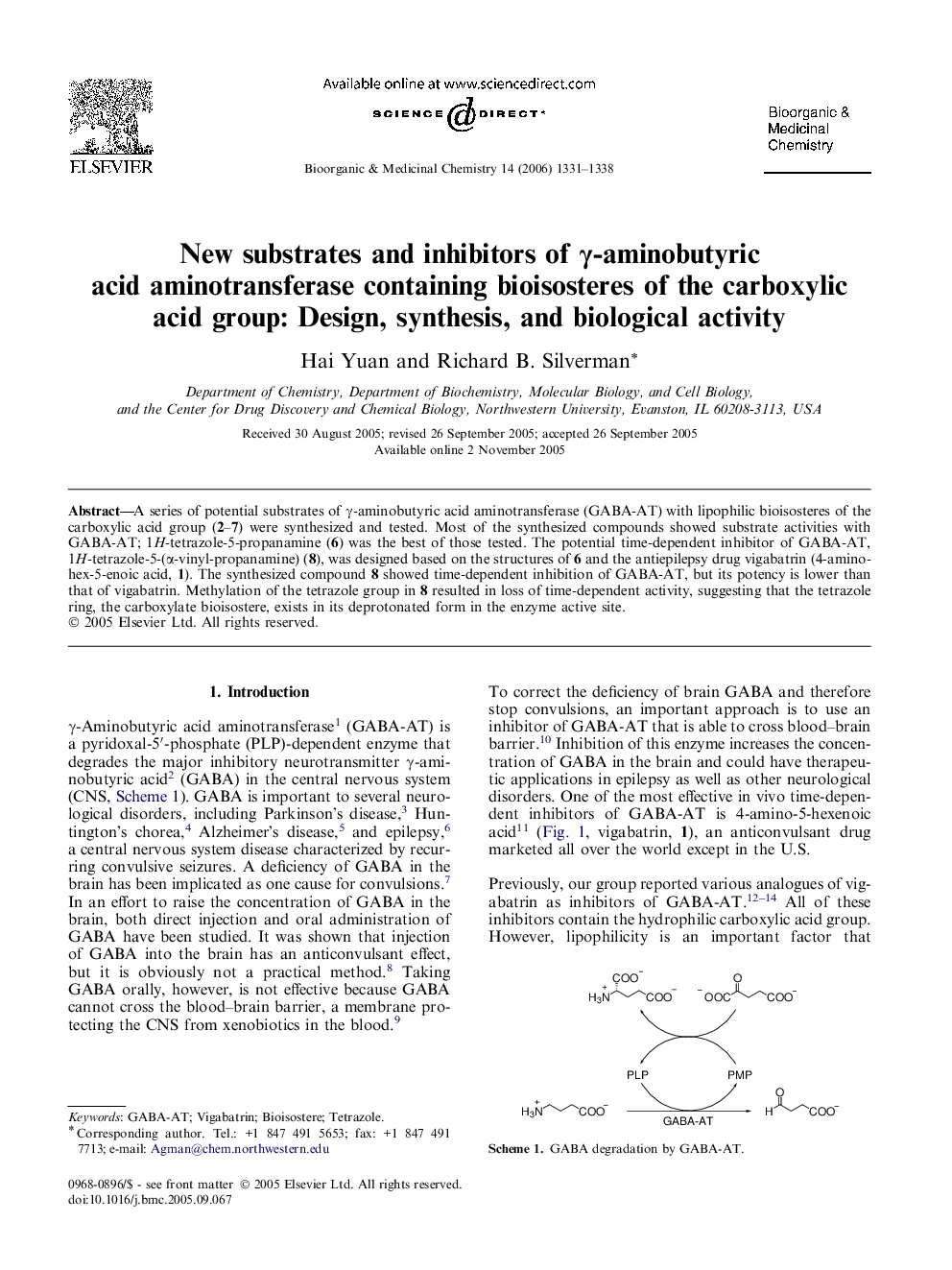
A series of potential substrates of γ-aminobutyric acid aminotransferase (GABA-AT) with lipophilic bioisosteres of the carboxylic acid group (2–7) were synthesized and tested. Most of the synthesized compounds showed substrate activities with GABA-AT; 1H-tetrazole-5-propanamine (6) was the best of those tested. The potential time-dependent inhibitor of GABA-AT, 1H-tetrazole-5-(α-vinyl-propanamine) (8), was designed based on the structures of 6 and the antiepilepsy drug vigabatrin (4-aminohex-5-enoic acid, 1). The synthesized compound 8 showed time-dependent inhibition of GABA-AT, but its potency is lower than that of vigabatrin. Methylation of the tetrazole group in 8 resulted in loss of time-dependent activity, suggesting that the tetrazole ring, the carboxylate bioisostere, exists in its deprotonated form in the enzyme active site.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 14, Issue 5, 1 March 2006, Pages 1331–1338