| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1362671 | 981493 | 2006 | 6 صفحه PDF | دانلود رایگان |
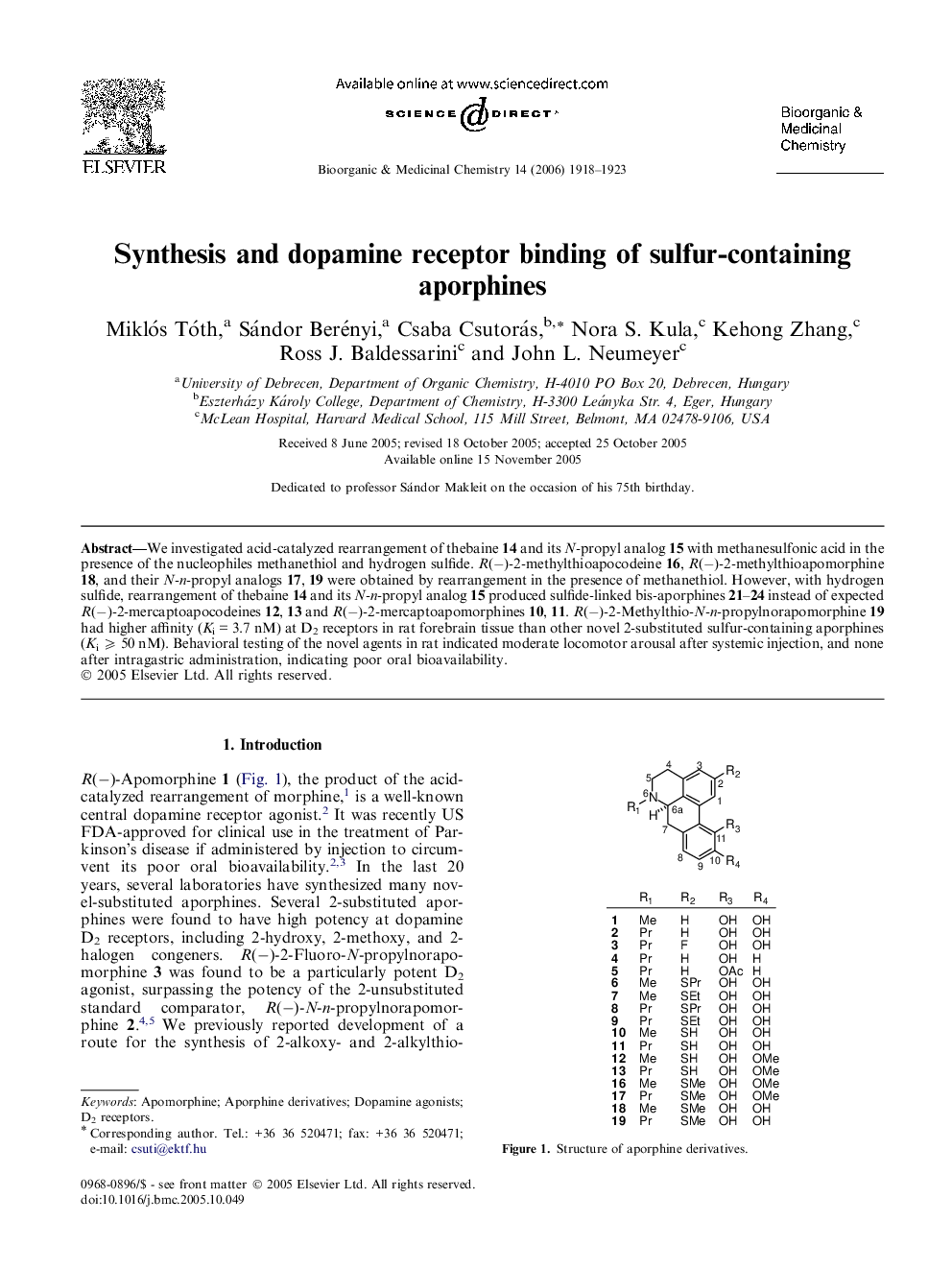
We investigated acid-catalyzed rearrangement of thebaine 14 and its N-propyl analog 15 with methanesulfonic acid in the presence of the nucleophiles methanethiol and hydrogen sulfide. R(−)-2-methylthioapocodeine 16, R(−)-2-methylthioapomorphine 18, and their N-n-propyl analogs 17, 19 were obtained by rearrangement in the presence of methanethiol. However, with hydrogen sulfide, rearrangement of thebaine 14 and its N-n-propyl analog 15 produced sulfide-linked bis-aporphines 21–24 instead of expected R(−)-2-mercaptoapocodeines 12, 13 and R(−)-2-mercaptoapomorphines 10, 11. R(−)-2-Methylthio-N-n-propylnorapomorphine 19 had higher affinity (Ki = 3.7 nM) at D2 receptors in rat forebrain tissue than other novel 2-substituted sulfur-containing aporphines (Ki ⩾ 50 nM). Behavioral testing of the novel agents in rat indicated moderate locomotor arousal after systemic injection, and none after intragastric administration, indicating poor oral bioavailability.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 14, Issue 6, 15 March 2006, Pages 1918–1923