| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1364372 | 981535 | 2008 | 4 صفحه PDF | دانلود رایگان |
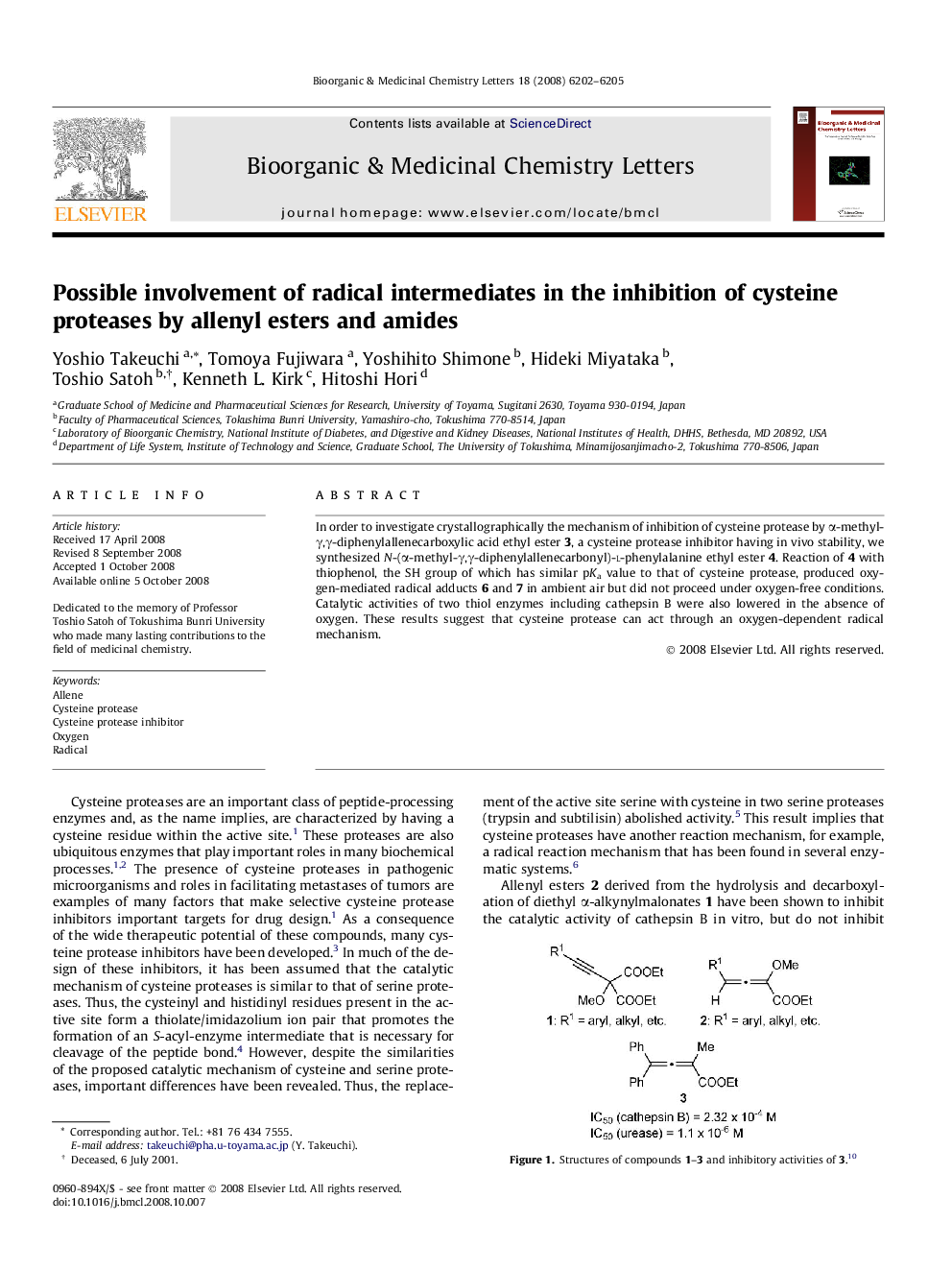
In order to investigate crystallographically the mechanism of inhibition of cysteine protease by α-methyl-γ,γ-diphenylallenecarboxylic acid ethyl ester 3, a cysteine protease inhibitor having in vivo stability, we synthesized N-(α-methyl-γ,γ-diphenylallenecarbonyl)-l-phenylalanine ethyl ester 4. Reaction of 4 with thiophenol, the SH group of which has similar pKa value to that of cysteine protease, produced oxygen-mediated radical adducts 6 and 7 in ambient air but did not proceed under oxygen-free conditions. Catalytic activities of two thiol enzymes including cathepsin B were also lowered in the absence of oxygen. These results suggest that cysteine protease can act through an oxygen-dependent radical mechanism.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 18, Issue 23, 1 December 2008, Pages 6202–6205