| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1365933 | 981577 | 2006 | 10 صفحه PDF | دانلود رایگان |
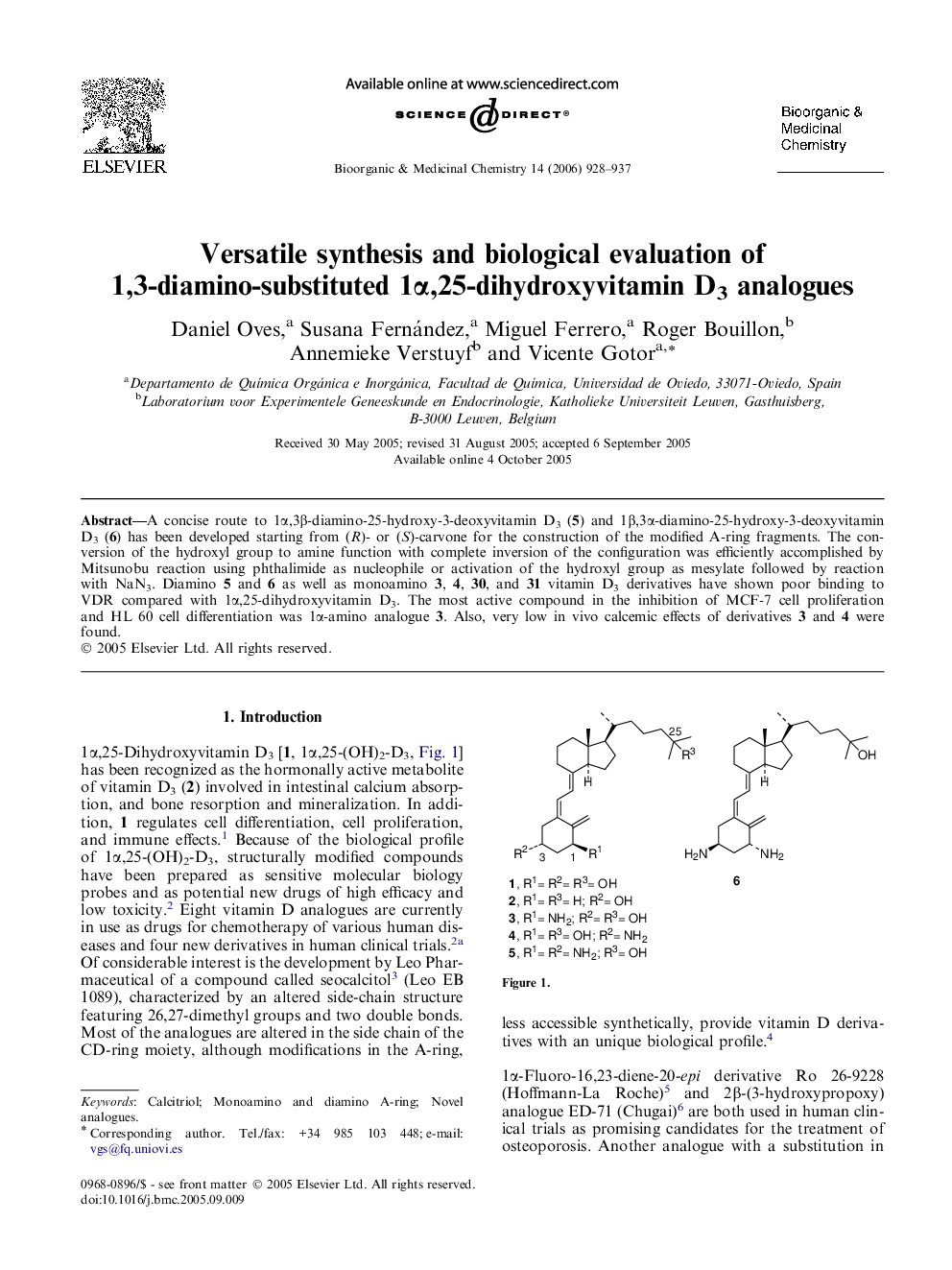
A concise route to 1α,3β-diamino-25-hydroxy-3-deoxyvitamin D3 (5) and 1β,3α-diamino-25-hydroxy-3-deoxyvitamin D3 (6) has been developed starting from (R)- or (S)-carvone for the construction of the modified A-ring fragments. The conversion of the hydroxyl group to amine function with complete inversion of the configuration was efficiently accomplished by Mitsunobu reaction using phthalimide as nucleophile or activation of the hydroxyl group as mesylate followed by reaction with NaN3. Diamino 5 and 6 as well as monoamino 3, 4, 30, and 31 vitamin D3 derivatives have shown poor binding to VDR compared with 1α,25-dihydroxyvitamin D3. The most active compound in the inhibition of MCF-7 cell proliferation and HL 60 cell differentiation was 1α-amino analogue 3. Also, very low in vivo calcemic effects of derivatives 3 and 4 were found.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 14, Issue 4, 15 February 2006, Pages 928–937