| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1366980 | 981611 | 2007 | 4 صفحه PDF | دانلود رایگان |
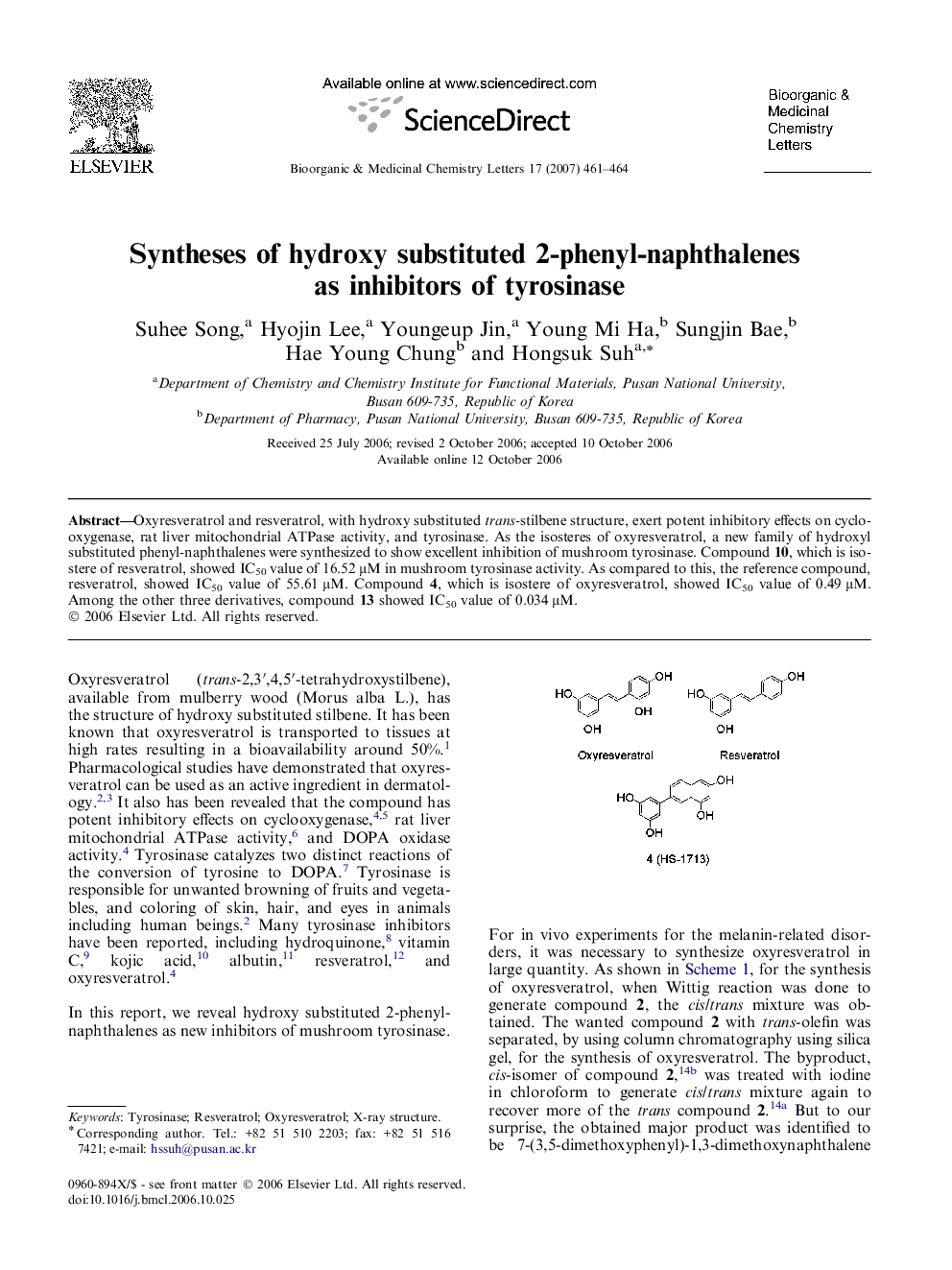
Oxyresveratrol and resveratrol, with hydroxy substituted trans-stilbene structure, exert potent inhibitory effects on cyclooxygenase, rat liver mitochondrial ATPase activity, and tyrosinase. As the isosteres of oxyresveratrol, a new family of hydroxyl substituted phenyl-naphthalenes were synthesized to show excellent inhibition of mushroom tyrosinase. Compound 10, which is isostere of resveratrol, showed IC50 value of 16.52 μM in mushroom tyrosinase activity. As compared to this, the reference compound, resveratrol, showed IC50 value of 55.61 μM. Compound 4, which is isostere of oxyresveratrol, showed IC50 value of 0.49 μM. Among the other three derivatives, compound 13 showed IC50 value of 0.034 μM.
Isosteres of oxyresveratrol and resveratrol and their derivatives with 2-phenyl-naphthalene template were synthesized and evaluated as tyrosinase inhibitors.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 17, Issue 2, 15 January 2007, Pages 461–464