| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1370234 | 981814 | 2011 | 7 صفحه PDF | دانلود رایگان |
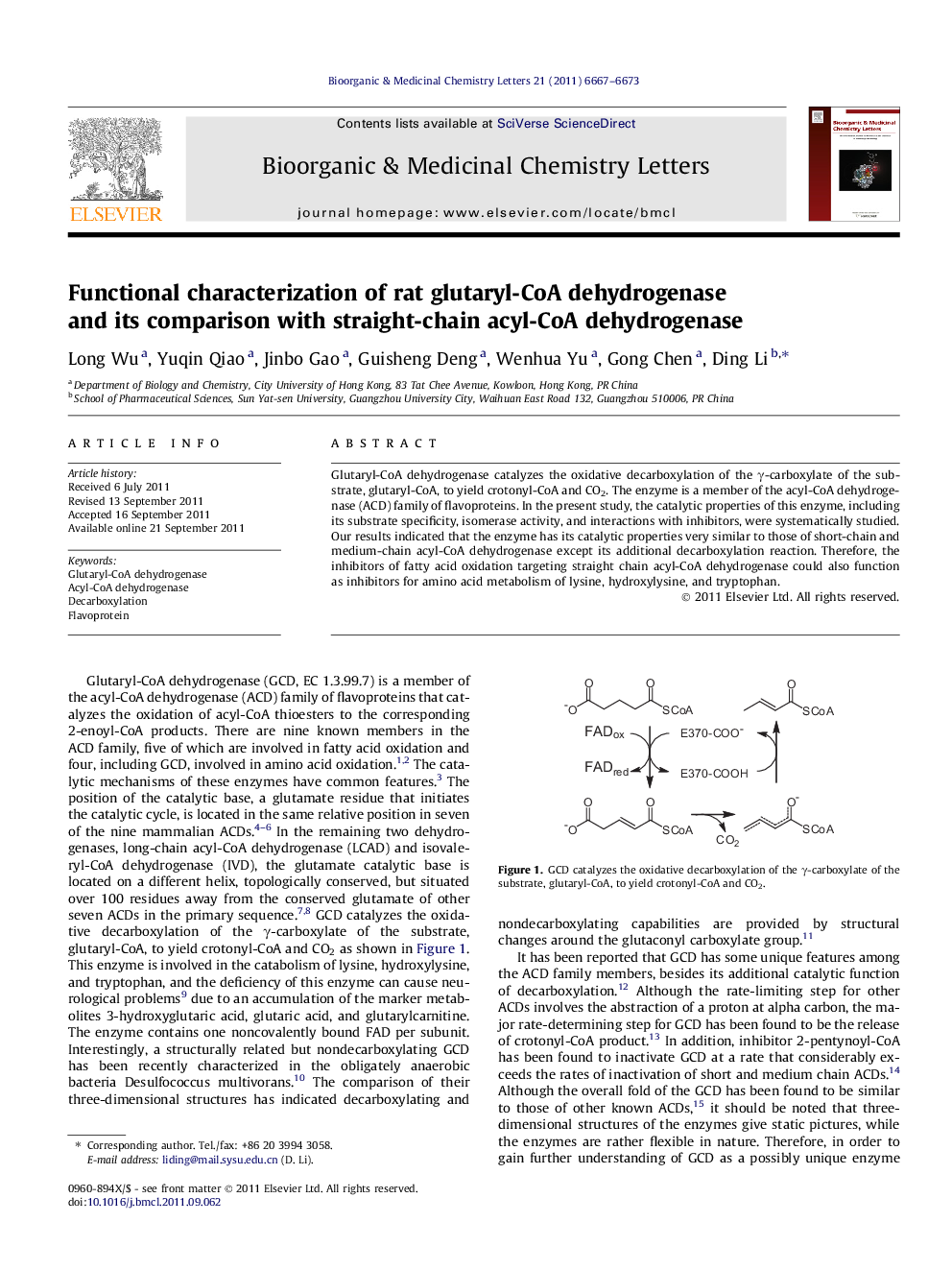
Glutaryl-CoA dehydrogenase catalyzes the oxidative decarboxylation of the γ-carboxylate of the substrate, glutaryl-CoA, to yield crotonyl-CoA and CO2. The enzyme is a member of the acyl-CoA dehydrogenase (ACD) family of flavoproteins. In the present study, the catalytic properties of this enzyme, including its substrate specificity, isomerase activity, and interactions with inhibitors, were systematically studied. Our results indicated that the enzyme has its catalytic properties very similar to those of short-chain and medium-chain acyl-CoA dehydrogenase except its additional decarboxylation reaction. Therefore, the inhibitors of fatty acid oxidation targeting straight chain acyl-CoA dehydrogenase could also function as inhibitors for amino acid metabolism of lysine, hydroxylysine, and tryptophan.
The catalytic properties of glutaryl-CoA dehydrogenase, including its substrate specificity, isomerase activity, and interactions with inhibitors, were systematically studied.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 21, Issue 22, 15 November 2011, Pages 6667–6673