| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1371617 | 981849 | 2010 | 4 صفحه PDF | دانلود رایگان |
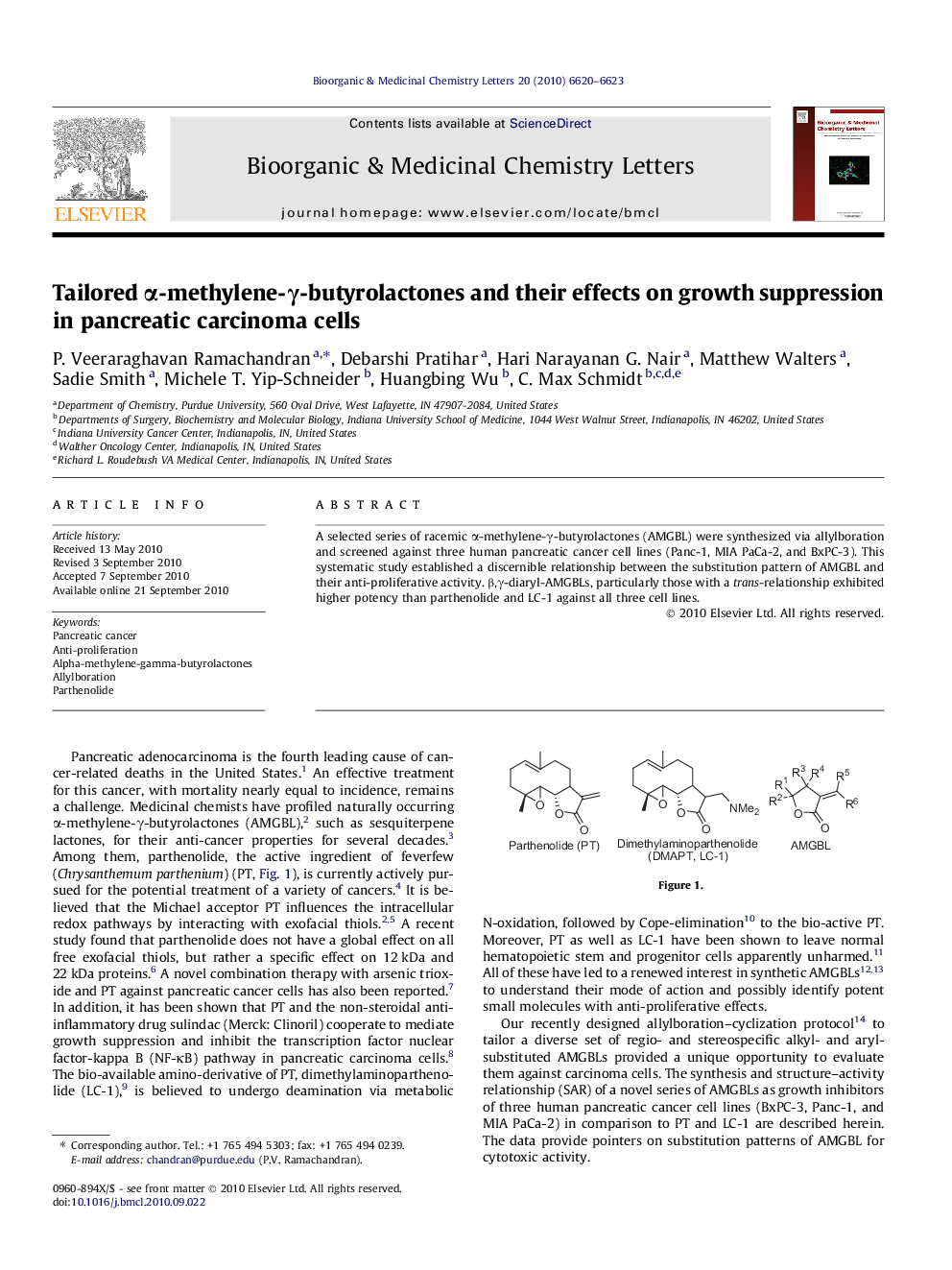
A selected series of racemic α-methylene-γ-butyrolactones (AMGBL) were synthesized via allylboration and screened against three human pancreatic cancer cell lines (Panc-1, MIA PaCa-2, and BxPC-3). This systematic study established a discernible relationship between the substitution pattern of AMGBL and their anti-proliferative activity. β,γ-diaryl-AMGBLs, particularly those with a trans-relationship exhibited higher potency than parthenolide and LC-1 against all three cell lines.
A selected series of α-methylene-γ-butyrolactones (AMGBL) were synthesized via allylboration and a discernible relationship between the substitution pattern and anti-proliferative activity was established by screening against three human pancreatic cancer cell lines; β,γ-diaryl-AMGBLs exhibited higher potency than parthenolide and LC-1.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 20, Issue 22, 15 November 2010, Pages 6620–6623