| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1372572 | 981871 | 2011 | 4 صفحه PDF | دانلود رایگان |
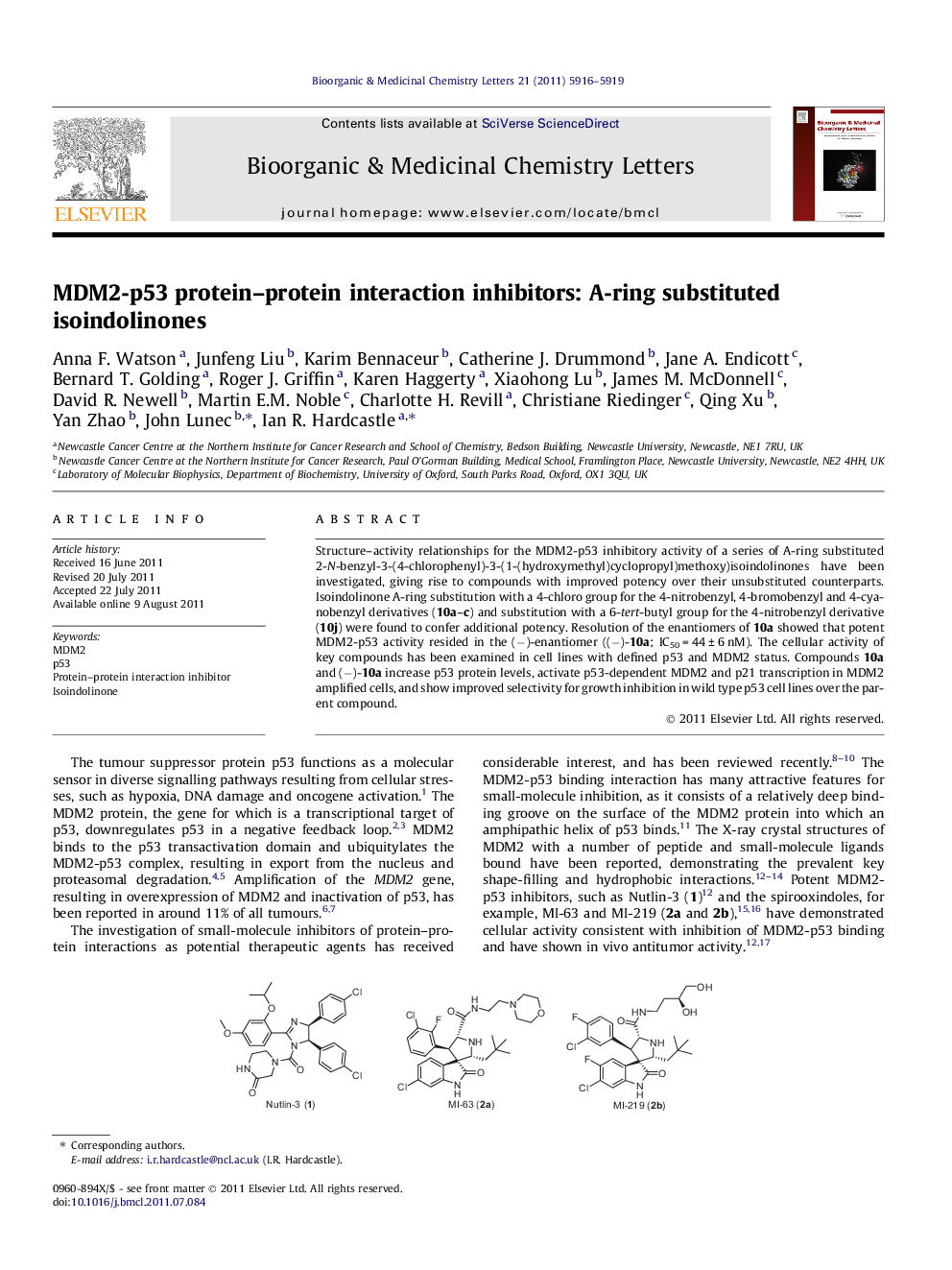
Structure–activity relationships for the MDM2-p53 inhibitory activity of a series of A-ring substituted 2-N-benzyl-3-(4-chlorophenyl)-3-(1-(hydroxymethyl)cyclopropyl)methoxy)isoindolinones have been investigated, giving rise to compounds with improved potency over their unsubstituted counterparts. Isoindolinone A-ring substitution with a 4-chloro group for the 4-nitrobenzyl, 4-bromobenzyl and 4-cyanobenzyl derivatives (10a–c) and substitution with a 6-tert-butyl group for the 4-nitrobenzyl derivative (10j) were found to confer additional potency. Resolution of the enantiomers of 10a showed that potent MDM2-p53 activity resided in the (−)-enantiomer ((−)-10a; IC50 = 44 ± 6 nM). The cellular activity of key compounds has been examined in cell lines with defined p53 and MDM2 status. Compounds 10a and (−)-10a increase p53 protein levels, activate p53-dependent MDM2 and p21 transcription in MDM2 amplified cells, and show improved selectivity for growth inhibition in wild type p53 cell lines over the parent compound.
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 21, Issue 19, 1 October 2011, Pages 5916–5919