| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1373047 | 981888 | 2008 | 4 صفحه PDF | دانلود رایگان |
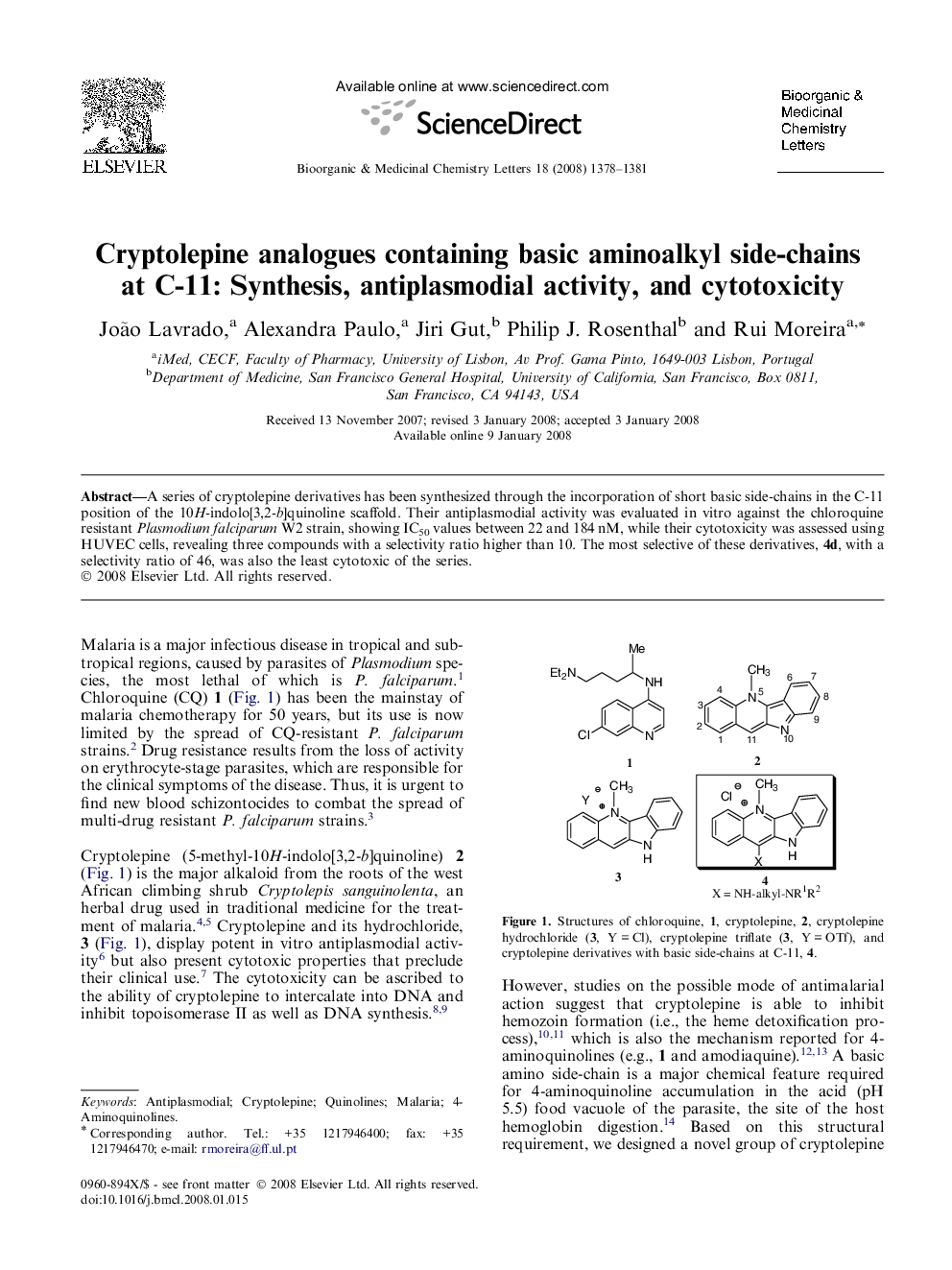
A series of cryptolepine derivatives has been synthesized through the incorporation of short basic side-chains in the C-11 position of the 10H-indolo[3,2-b]quinoline scaffold. Their antiplasmodial activity was evaluated in vitro against the chloroquine resistant Plasmodium falciparum W2 strain, showing IC50 values between 22 and 184 nM, while their cytotoxicity was assessed using HUVEC cells, revealing three compounds with a selectivity ratio higher than 10. The most selective of these derivatives, 4d, with a selectivity ratio of 46, was also the least cytotoxic of the series.
Cryptolepine derivatives containing a basic short chain at position C-11 present IC50 values against the chloroquine-resistant Plasmodium falciparum W2 strain ranging from 22 to 184 nM.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 18, Issue 4, 15 February 2008, Pages 1378–1381