| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1376634 | 981962 | 2008 | 5 صفحه PDF | دانلود رایگان |
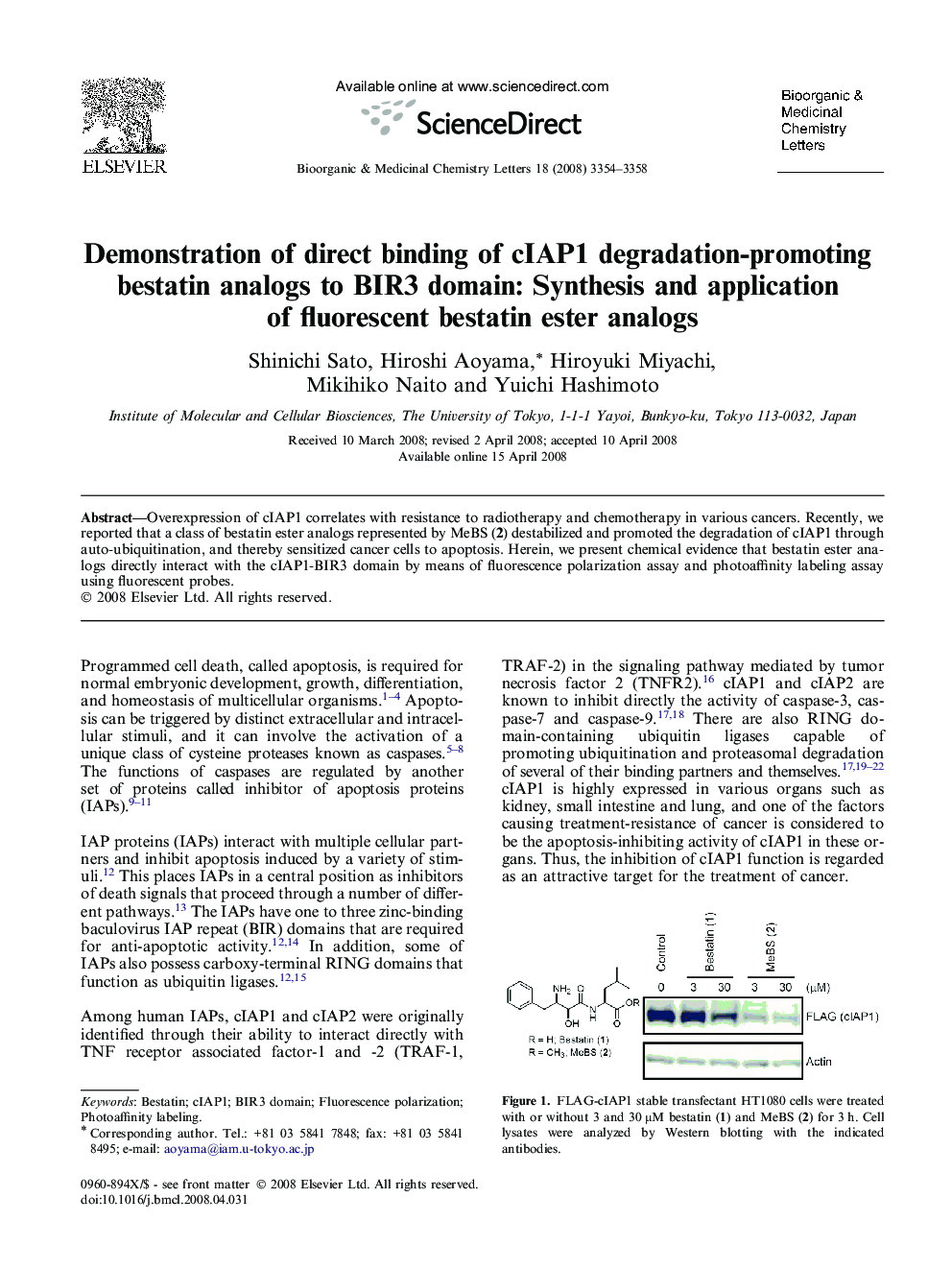
Overexpression of cIAP1 correlates with resistance to radiotherapy and chemotherapy in various cancers. Recently, we reported that a class of bestatin ester analogs represented by MeBS (2) destabilized and promoted the degradation of cIAP1 through auto-ubiquitination, and thereby sensitized cancer cells to apoptosis. Herein, we present chemical evidence that bestatin ester analogs directly interact with the cIAP1-BIR3 domain by means of fluorescence polarization assay and photoaffinity labeling assay using fluorescent probes.
Fluorescent bestatin ester analogs 3 and 4 were designed and synthesized. Direct binding of cIAP1-BIR3 domain protein and these probes was observed in fluorescence polarization assay and photoaffinity labeling assay.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 18, Issue 11, 1 June 2008, Pages 3354–3358