| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1390491 | 983088 | 2011 | 10 صفحه PDF | دانلود رایگان |
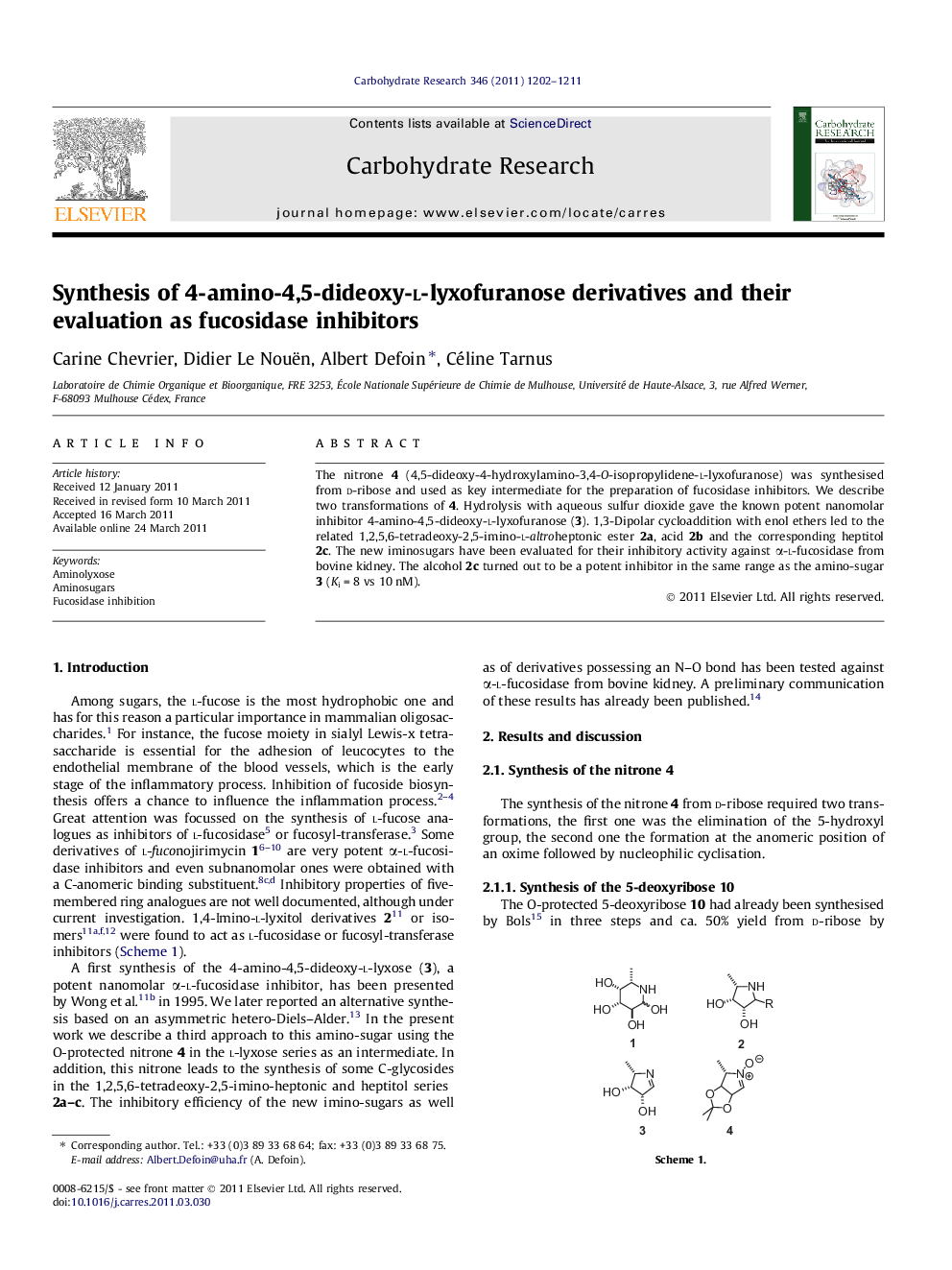
The nitrone 4 (4,5-dideoxy-4-hydroxylamino-3,4-O-isopropylidene-l-lyxofuranose) was synthesised from d-ribose and used as key intermediate for the preparation of fucosidase inhibitors. We describe two transformations of 4. Hydrolysis with aqueous sulfur dioxide gave the known potent nanomolar inhibitor 4-amino-4,5-dideoxy-l-lyxofuranose (3). 1,3-Dipolar cycloaddition with enol ethers led to the related 1,2,5,6-tetradeoxy-2,5-imino-l-altroheptonic ester 2a, acid 2b and the corresponding heptitol 2c. The new iminosugars have been evaluated for their inhibitory activity against α-l-fucosidase from bovine kidney. The alcohol 2c turned out to be a potent inhibitor in the same range as the amino-sugar 3 (Ki = 8 vs 10 nM).
The nitrone 4 was synthesised from d-ribose. We described its transformations into fucosidase inhibitors, particularly the known 4-amino-l-lyxose 3 and the C-β-hydroxy-ethyl derivative 2c (Ki = 10 and 8 nM, respectively).Figure optionsDownload as PowerPoint slide
Journal: Carbohydrate Research - Volume 346, Issue 10, 15 July 2011, Pages 1202–1211