| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1391757 | 983632 | 2013 | 11 صفحه PDF | دانلود رایگان |
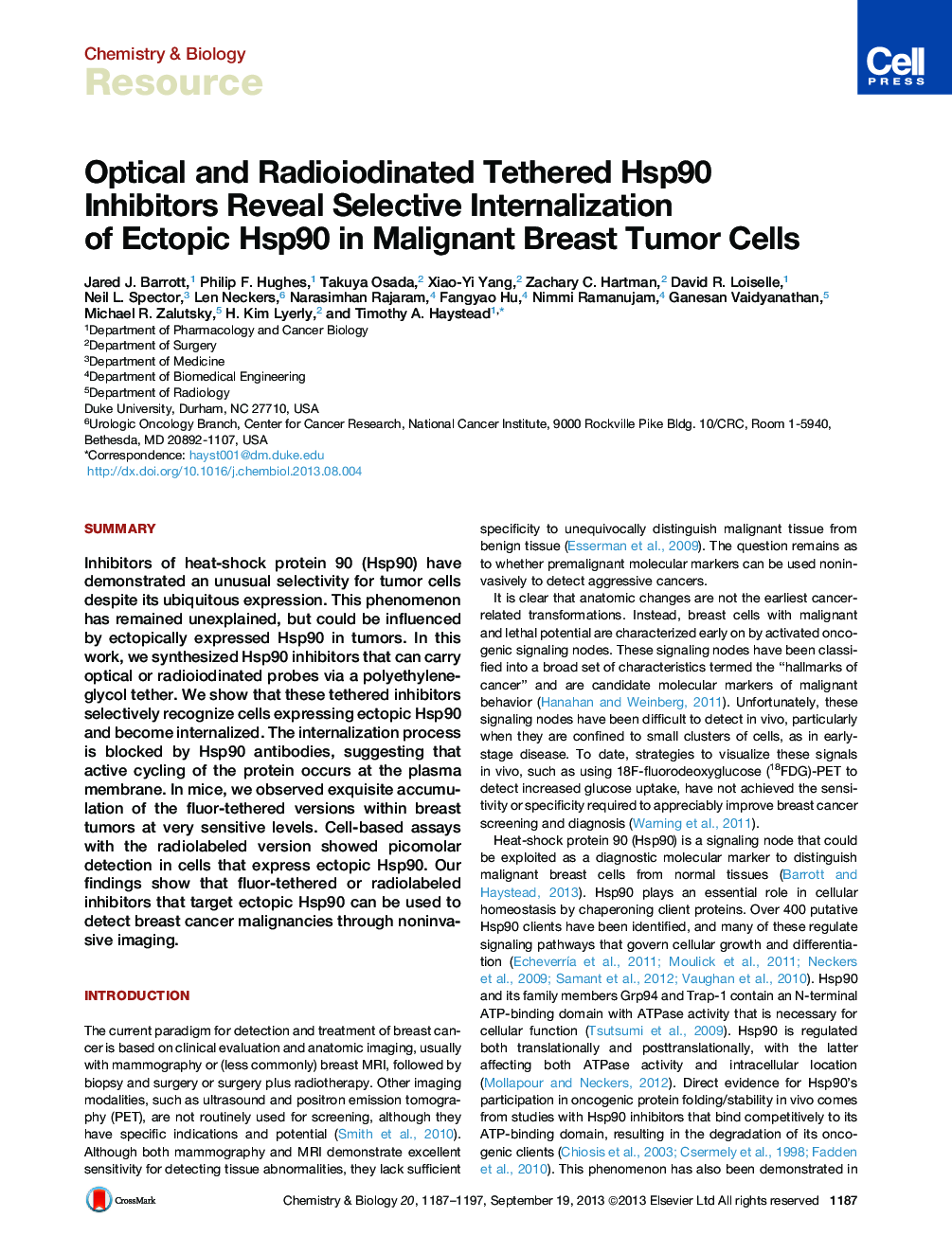
• Tethered Hsp90 inhibitors are selectively internalized in cancer cells
• Ectopic Hsp90 facilitates probe internalization and is unique to tumors
• Nonmalignant tissues contain cytoplasmic Hsp90 that binds inhibitors after lysis
• 125I and nIR tethered inhibitors can be detected at picomolar amounts and 100,000 cells
SummaryInhibitors of heat-shock protein 90 (Hsp90) have demonstrated an unusual selectivity for tumor cells despite its ubiquitous expression. This phenomenon has remained unexplained, but could be influenced by ectopically expressed Hsp90 in tumors. In this work, we synthesized Hsp90 inhibitors that can carry optical or radioiodinated probes via a polyethyleneglycol tether. We show that these tethered inhibitors selectively recognize cells expressing ectopic Hsp90 and become internalized. The internalization process is blocked by Hsp90 antibodies, suggesting that active cycling of the protein occurs at the plasma membrane. In mice, we observed exquisite accumulation of the fluor-tethered versions within breast tumors at very sensitive levels. Cell-based assays with the radiolabeled version showed picomolar detection in cells that express ectopic Hsp90. Our findings show that fluor-tethered or radiolabeled inhibitors that target ectopic Hsp90 can be used to detect breast cancer malignancies through noninvasive imaging.
Graphical AbstractFigure optionsDownload high-quality image (232 K)Download as PowerPoint slide
Journal: - Volume 20, Issue 9, 19 September 2013, Pages 1187–1197