| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145340 | 456338 | 2016 | 12 صفحه PDF | دانلود رایگان |
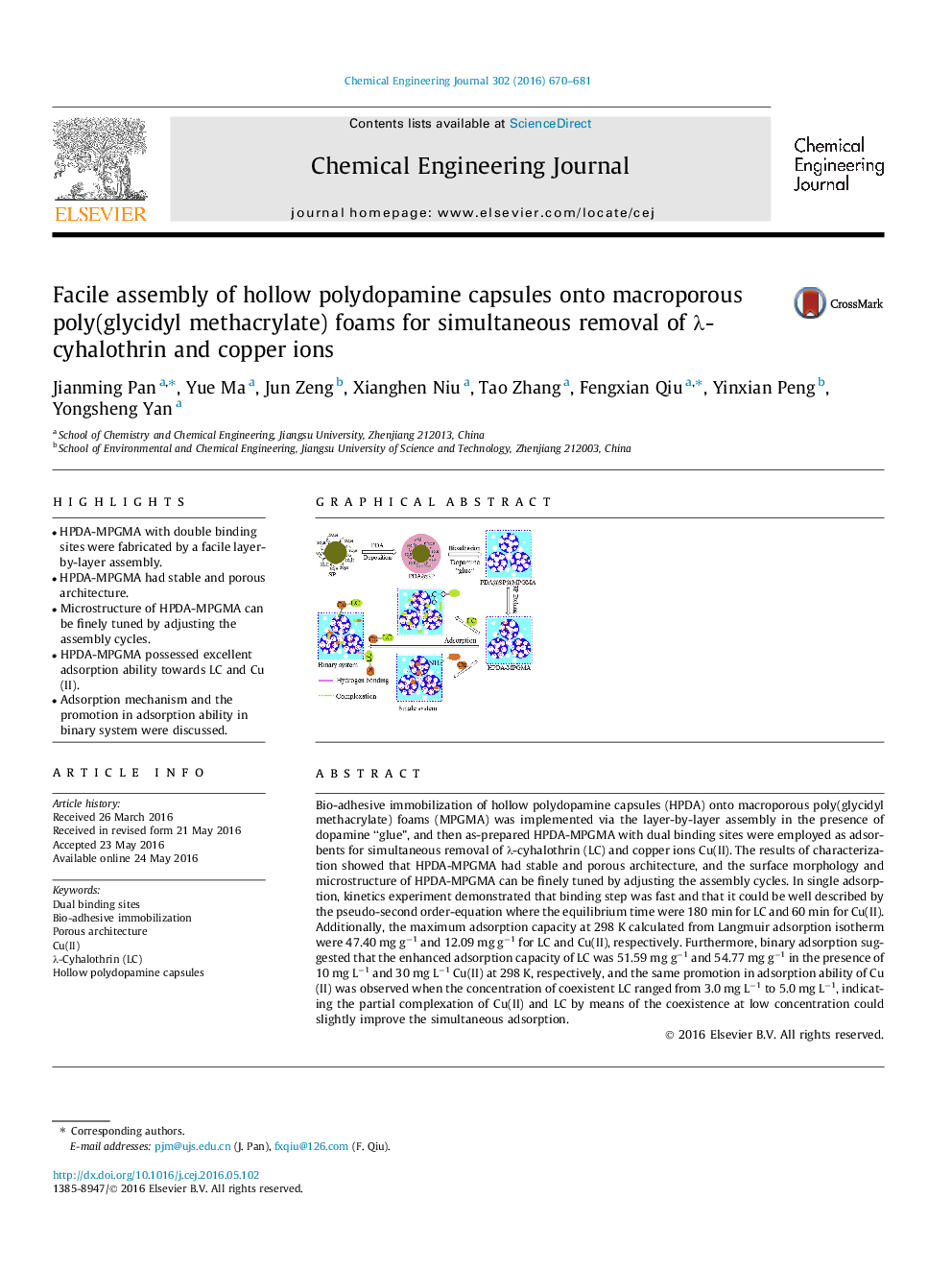
• HPDA-MPGMA with double binding sites were fabricated by a facile layer-by-layer assembly.
• HPDA-MPGMA had stable and porous architecture.
• Microstructure of HPDA-MPGMA can be finely tuned by adjusting the assembly cycles.
• HPDA-MPGMA possessed excellent adsorption ability towards LC and Cu(II).
• Adsorption mechanism and the promotion in adsorption ability in binary system were discussed.
Bio-adhesive immobilization of hollow polydopamine capsules (HPDA) onto macroporous poly(glycidyl methacrylate) foams (MPGMA) was implemented via the layer-by-layer assembly in the presence of dopamine “glue”, and then as-prepared HPDA-MPGMA with dual binding sites were employed as adsorbents for simultaneous removal of λ-cyhalothrin (LC) and copper ions Cu(II). The results of characterization showed that HPDA-MPGMA had stable and porous architecture, and the surface morphology and microstructure of HPDA-MPGMA can be finely tuned by adjusting the assembly cycles. In single adsorption, kinetics experiment demonstrated that binding step was fast and that it could be well described by the pseudo-second order-equation where the equilibrium time were 180 min for LC and 60 min for Cu(II). Additionally, the maximum adsorption capacity at 298 K calculated from Langmuir adsorption isotherm were 47.40 mg g−1 and 12.09 mg g−1 for LC and Cu(II), respectively. Furthermore, binary adsorption suggested that the enhanced adsorption capacity of LC was 51.59 mg g−1 and 54.77 mg g−1 in the presence of 10 mg L−1 and 30 mg L−1 Cu(II) at 298 K, respectively, and the same promotion in adsorption ability of Cu(II) was observed when the concentration of coexistent LC ranged from 3.0 mg L−1 to 5.0 mg L−1, indicating the partial complexation of Cu(II) and LC by means of the coexistence at low concentration could slightly improve the simultaneous adsorption.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 302, 15 October 2016, Pages 670–681