| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145540 | 456343 | 2016 | 8 صفحه PDF | دانلود رایگان |
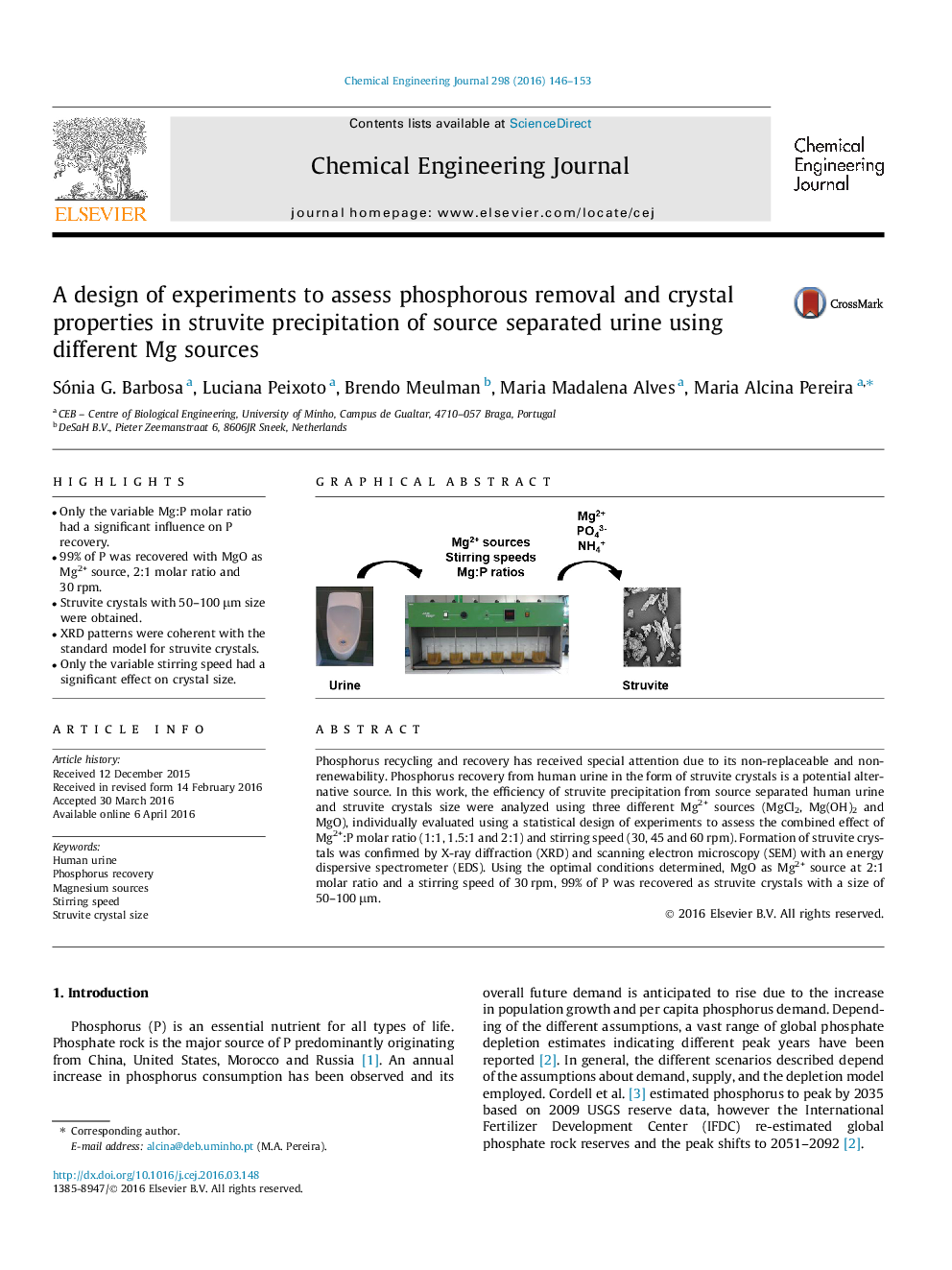
• Only the variable Mg:P molar ratio had a significant influence on P recovery.
• 99% of P was recovered with MgO as Mg2+ source, 2:1 molar ratio and 30 rpm.
• Struvite crystals with 50–100 μm size were obtained.
• XRD patterns were coherent with the standard model for struvite crystals.
• Only the variable stirring speed had a significant effect on crystal size.
Phosphorus recycling and recovery has received special attention due to its non-replaceable and non-renewability. Phosphorus recovery from human urine in the form of struvite crystals is a potential alternative source. In this work, the efficiency of struvite precipitation from source separated human urine and struvite crystals size were analyzed using three different Mg2+ sources (MgCl2, Mg(OH)2 and MgO), individually evaluated using a statistical design of experiments to assess the combined effect of Mg2+:P molar ratio (1:1, 1.5:1 and 2:1) and stirring speed (30, 45 and 60 rpm). Formation of struvite crystals was confirmed by X-ray diffraction (XRD) and scanning electron microscopy (SEM) with an energy dispersive spectrometer (EDS). Using the optimal conditions determined, MgO as Mg2+ source at 2:1 molar ratio and a stirring speed of 30 rpm, 99% of P was recovered as struvite crystals with a size of 50–100 μm.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 298, 15 August 2016, Pages 146–153