| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145841 | 456352 | 2016 | 10 صفحه PDF | دانلود رایگان |
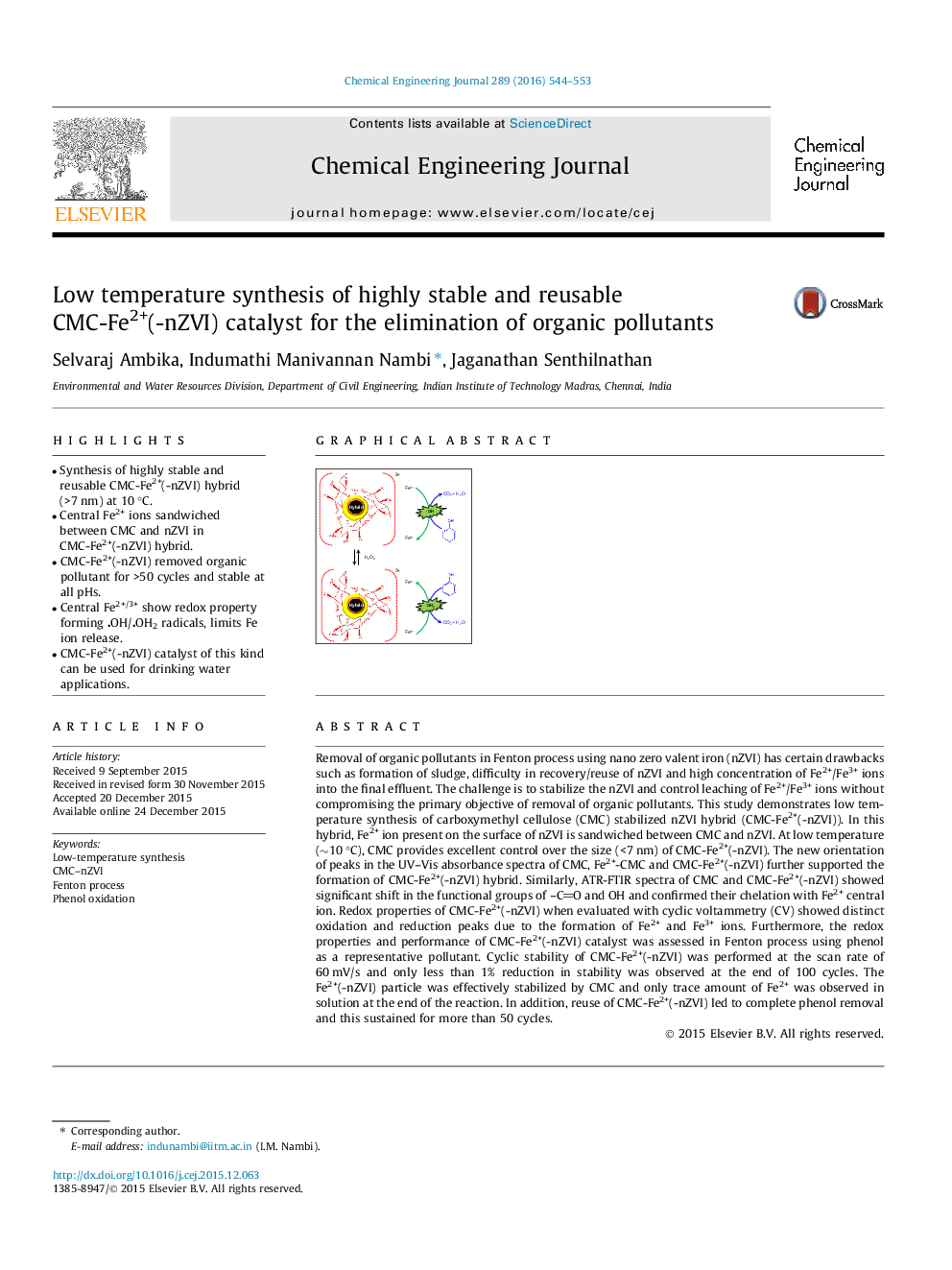
• Synthesis of highly stable and reusable CMC-Fe2+(-nZVI) hybrid (>7 nm) at 10 °C.
• Central Fe2+ ions sandwiched between CMC and nZVI in CMC-Fe2+(-nZVI) hybrid.
• CMC-Fe2+(-nZVI) removed organic pollutant for >50 cycles and stable at all pHs.
• Central Fe2+/3+ show redox property forming OH/OH2 radicals, limits Fe ion release.
• CMC-Fe2+(-nZVI) catalyst of this kind can be used for drinking water applications.
Removal of organic pollutants in Fenton process using nano zero valent iron (nZVI) has certain drawbacks such as formation of sludge, difficulty in recovery/reuse of nZVI and high concentration of Fe2+/Fe3+ ions into the final effluent. The challenge is to stabilize the nZVI and control leaching of Fe2+/Fe3+ ions without compromising the primary objective of removal of organic pollutants. This study demonstrates low temperature synthesis of carboxymethyl cellulose (CMC) stabilized nZVI hybrid (CMC-Fe2+(-nZVI)). In this hybrid, Fe2+ ion present on the surface of nZVI is sandwiched between CMC and nZVI. At low temperature (∼10 °C), CMC provides excellent control over the size (<7 nm) of CMC-Fe2+(-nZVI). The new orientation of peaks in the UV–Vis absorbance spectra of CMC, Fe2+-CMC and CMC-Fe2+(-nZVI) further supported the formation of CMC-Fe2+(-nZVI) hybrid. Similarly, ATR-FTIR spectra of CMC and CMC-Fe2+(-nZVI) showed significant shift in the functional groups of –CO and OH and confirmed their chelation with Fe2+ central ion. Redox properties of CMC-Fe2+(-nZVI) when evaluated with cyclic voltammetry (CV) showed distinct oxidation and reduction peaks due to the formation of Fe2+ and Fe3+ ions. Furthermore, the redox properties and performance of CMC-Fe2+(-nZVI) catalyst was assessed in Fenton process using phenol as a representative pollutant. Cyclic stability of CMC-Fe2+(-nZVI) was performed at the scan rate of 60 mV/s and only less than 1% reduction in stability was observed at the end of 100 cycles. The Fe2+(-nZVI) particle was effectively stabilized by CMC and only trace amount of Fe2+ was observed in solution at the end of the reaction. In addition, reuse of CMC-Fe2+(-nZVI) led to complete phenol removal and this sustained for more than 50 cycles.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 289, 1 April 2016, Pages 544–553