| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146172 | 456363 | 2015 | 8 صفحه PDF | دانلود رایگان |
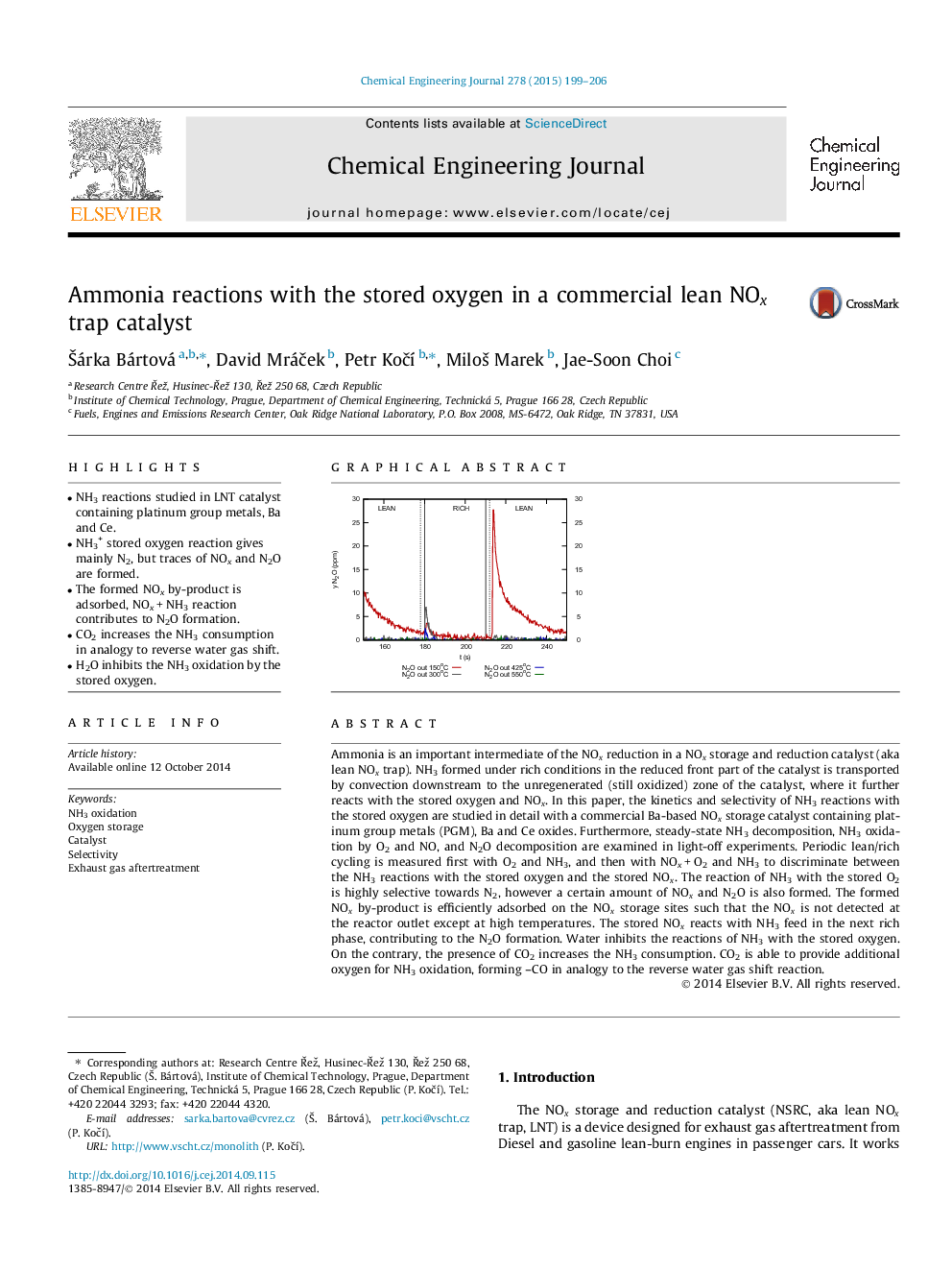
• NH3 reactions studied in LNT catalyst containing platinum group metals, Ba and Ce.
• NH3+ stored oxygen reaction gives mainly N2, but traces of NOx and N2O are formed.
• The formed NOx by-product is adsorbed, NOx + NH3 reaction contributes to N2O formation.
• CO2 increases the NH3 consumption in analogy to reverse water gas shift.
• H2O inhibits the NH3 oxidation by the stored oxygen.
Ammonia is an important intermediate of the NOxNOx reduction in a NOxNOx storage and reduction catalyst (aka lean NOxNOx trap). NH3 formed under rich conditions in the reduced front part of the catalyst is transported by convection downstream to the unregenerated (still oxidized) zone of the catalyst, where it further reacts with the stored oxygen and NOxNOx. In this paper, the kinetics and selectivity of NH3 reactions with the stored oxygen are studied in detail with a commercial Ba-based NOxNOx storage catalyst containing platinum group metals (PGM), Ba and Ce oxides. Furthermore, steady-state NH3 decomposition, NH3 oxidation by O2 and NO, and N2O decomposition are examined in light-off experiments. Periodic lean/rich cycling is measured first with O2 and NH3, and then with NOx + O2 and NH3 to discriminate between the NH3 reactions with the stored oxygen and the stored NOxNOx. The reaction of NH3 with the stored O2 is highly selective towards N2, however a certain amount of NOxNOx and N2O is also formed. The formed NOxNOx by-product is efficiently adsorbed on the NOxNOx storage sites such that the NOxNOx is not detected at the reactor outlet except at high temperatures. The stored NOxNOx reacts with NH3 feed in the next rich phase, contributing to the N2O formation. Water inhibits the reactions of NH3 with the stored oxygen. On the contrary, the presence of CO2 increases the NH3 consumption. CO2 is able to provide additional oxygen for NH3 oxidation, forming –CO in analogy to the reverse water gas shift reaction.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 278, 15 October 2015, Pages 199–206