| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146362 | 456368 | 2015 | 12 صفحه PDF | دانلود رایگان |
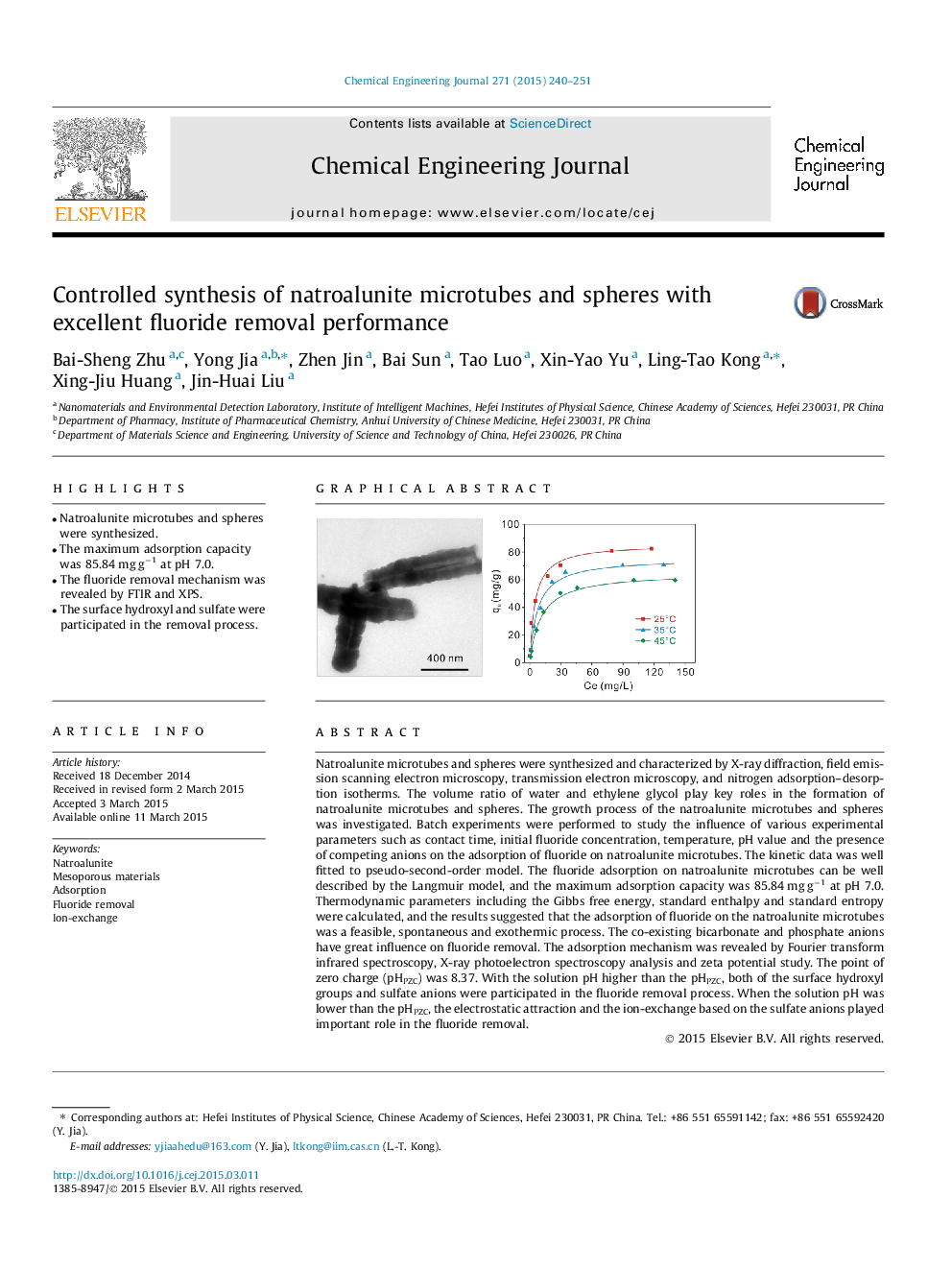
• Natroalunite microtubes and spheres were synthesized.
• The maximum adsorption capacity was 85.84 mg g−1 at pH 7.0.
• The fluoride removal mechanism was revealed by FTIR and XPS.
• The surface hydroxyl and sulfate were participated in the removal process.
Natroalunite microtubes and spheres were synthesized and characterized by X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy, and nitrogen adsorption–desorption isotherms. The volume ratio of water and ethylene glycol play key roles in the formation of natroalunite microtubes and spheres. The growth process of the natroalunite microtubes and spheres was investigated. Batch experiments were performed to study the influence of various experimental parameters such as contact time, initial fluoride concentration, temperature, pH value and the presence of competing anions on the adsorption of fluoride on natroalunite microtubes. The kinetic data was well fitted to pseudo-second-order model. The fluoride adsorption on natroalunite microtubes can be well described by the Langmuir model, and the maximum adsorption capacity was 85.84 mg g−1 at pH 7.0. Thermodynamic parameters including the Gibbs free energy, standard enthalpy and standard entropy were calculated, and the results suggested that the adsorption of fluoride on the natroalunite microtubes was a feasible, spontaneous and exothermic process. The co-existing bicarbonate and phosphate anions have great influence on fluoride removal. The adsorption mechanism was revealed by Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy analysis and zeta potential study. The point of zero charge (pHPZC) was 8.37. With the solution pH higher than the pHPZC, both of the surface hydroxyl groups and sulfate anions were participated in the fluoride removal process. When the solution pH was lower than the pHPZC, the electrostatic attraction and the ion-exchange based on the sulfate anions played important role in the fluoride removal.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 271, 1 July 2015, Pages 240–251