| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146715 | 456376 | 2015 | 9 صفحه PDF | دانلود رایگان |
• ZnS nanoflowers and nanorods exhibit enhanced photocatalytic activity.
• meta and para-dinitrobenzene is selectively photoreduced to (m&p)-nitroaniline.
• Hexagonal ZnS nanorods show higher photoactivity than cubic nanoflowers.
• Au and Pt deposition onto ZnS improves the yield of (m&p)-nitroaniline formation.
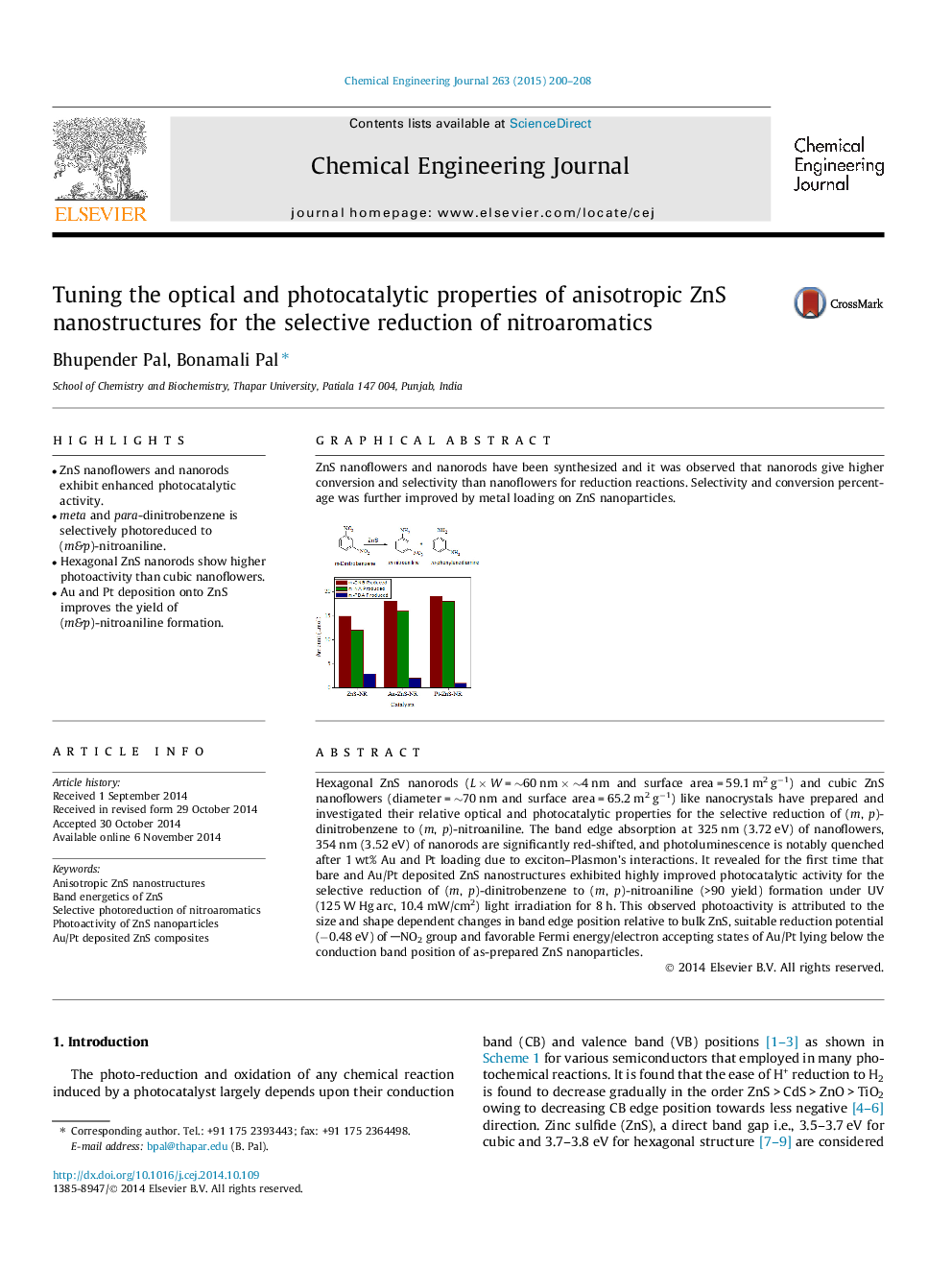
Hexagonal ZnS nanorods (L × W = ∼60 nm × ∼4 nm and surface area = 59.1 m2 g−1) and cubic ZnS nanoflowers (diameter = ∼70 nm and surface area = 65.2 m2 g−1) like nanocrystals have prepared and investigated their relative optical and photocatalytic properties for the selective reduction of (m, p)-dinitrobenzene to (m, p)-nitroaniline. The band edge absorption at 325 nm (3.72 eV) of nanoflowers, 354 nm (3.52 eV) of nanorods are significantly red-shifted, and photoluminescence is notably quenched after 1 wt% Au and Pt loading due to exciton–Plasmon’s interactions. It revealed for the first time that bare and Au/Pt deposited ZnS nanostructures exhibited highly improved photocatalytic activity for the selective reduction of (m, p)-dinitrobenzene to (m, p)-nitroaniline (>90 yield) formation under UV (125 W Hg arc, 10.4 mW/cm2) light irradiation for 8 h. This observed photoactivity is attributed to the size and shape dependent changes in band edge position relative to bulk ZnS, suitable reduction potential (−0.48 eV) of NO2 group and favorable Fermi energy/electron accepting states of Au/Pt lying below the conduction band position of as-prepared ZnS nanoparticles.
ZnS nanoflowers and nanorods have been synthesized and it was observed that nanorods give higher conversion and selectivity than nanoflowers for reduction reactions. Selectivity and conversion percentage was further improved by metal loading on ZnS nanoparticles.Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 263, 1 March 2015, Pages 200–208