| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146837 | 456379 | 2015 | 7 صفحه PDF | دانلود رایگان |
• Ca-loaded biomass was used to analyse the elimination mechanism of different heavy metals.
• Cadmium and lead interaction with biomass is mainly ruled by an ion exchange mechanism.
• Mercury elimination by alga biomass is a complex process including adsorption and reduction.
• SEM/EDS analysis confirms mercury reduction after batch sorption experiments.
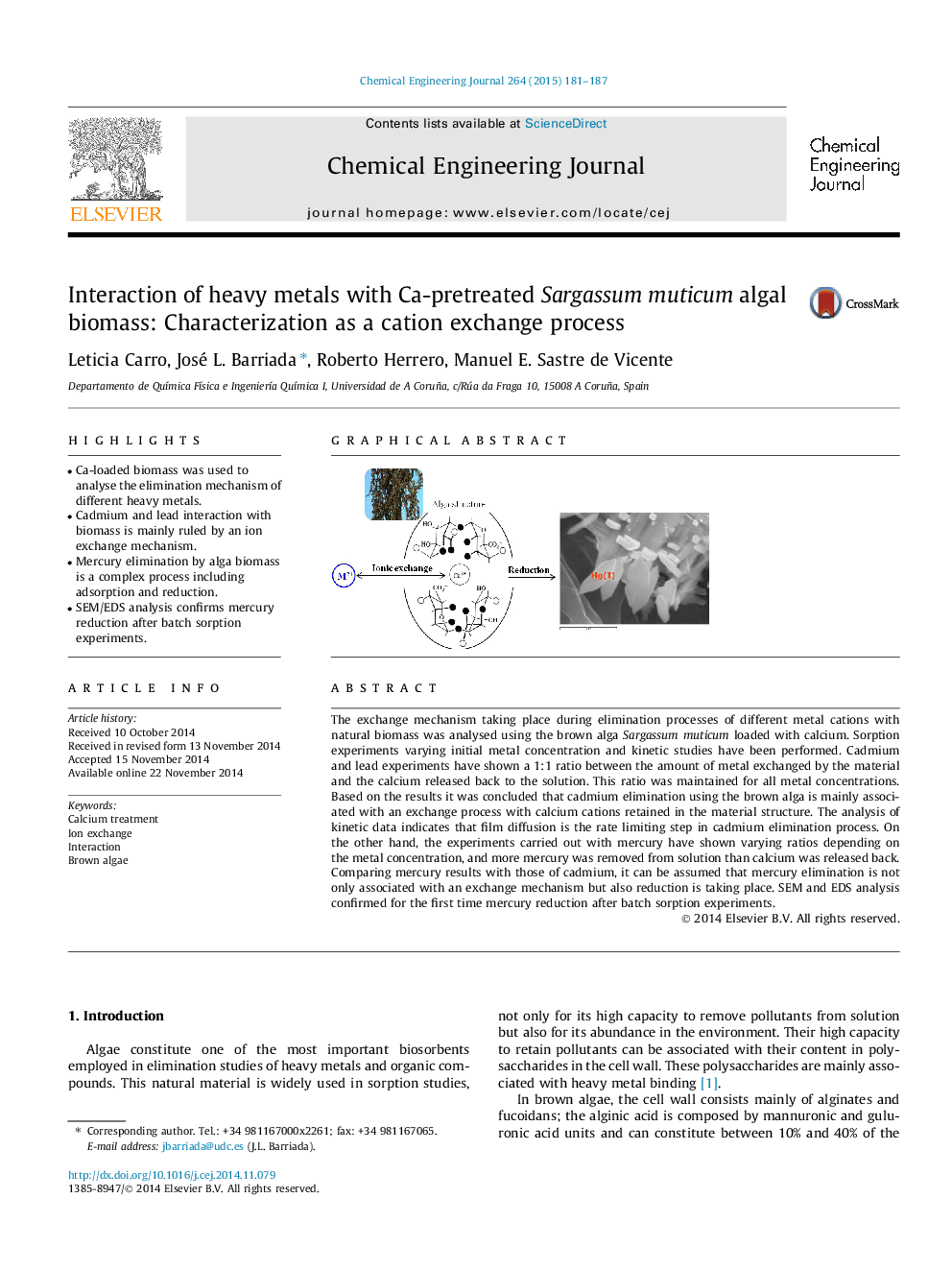
The exchange mechanism taking place during elimination processes of different metal cations with natural biomass was analysed using the brown alga Sargassum muticum loaded with calcium. Sorption experiments varying initial metal concentration and kinetic studies have been performed. Cadmium and lead experiments have shown a 1:1 ratio between the amount of metal exchanged by the material and the calcium released back to the solution. This ratio was maintained for all metal concentrations. Based on the results it was concluded that cadmium elimination using the brown alga is mainly associated with an exchange process with calcium cations retained in the material structure. The analysis of kinetic data indicates that film diffusion is the rate limiting step in cadmium elimination process. On the other hand, the experiments carried out with mercury have shown varying ratios depending on the metal concentration, and more mercury was removed from solution than calcium was released back. Comparing mercury results with those of cadmium, it can be assumed that mercury elimination is not only associated with an exchange mechanism but also reduction is taking place. SEM and EDS analysis confirmed for the first time mercury reduction after batch sorption experiments.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 264, 15 March 2015, Pages 181–187