| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146989 | 456384 | 2014 | 8 صفحه PDF | دانلود رایگان |
• CO2 adsorption kinetic study on pore-expanded mesoporous silica (MCM-41) material.
• Kinetic modeling under wide range of pressure and temperature conditions.
• Detailed kinetic analysis is carried out to predict kinetic behavior of CO2 uptake.
• Highlighted individual mechanistic steps that control the diffusion of CO2 uptake.
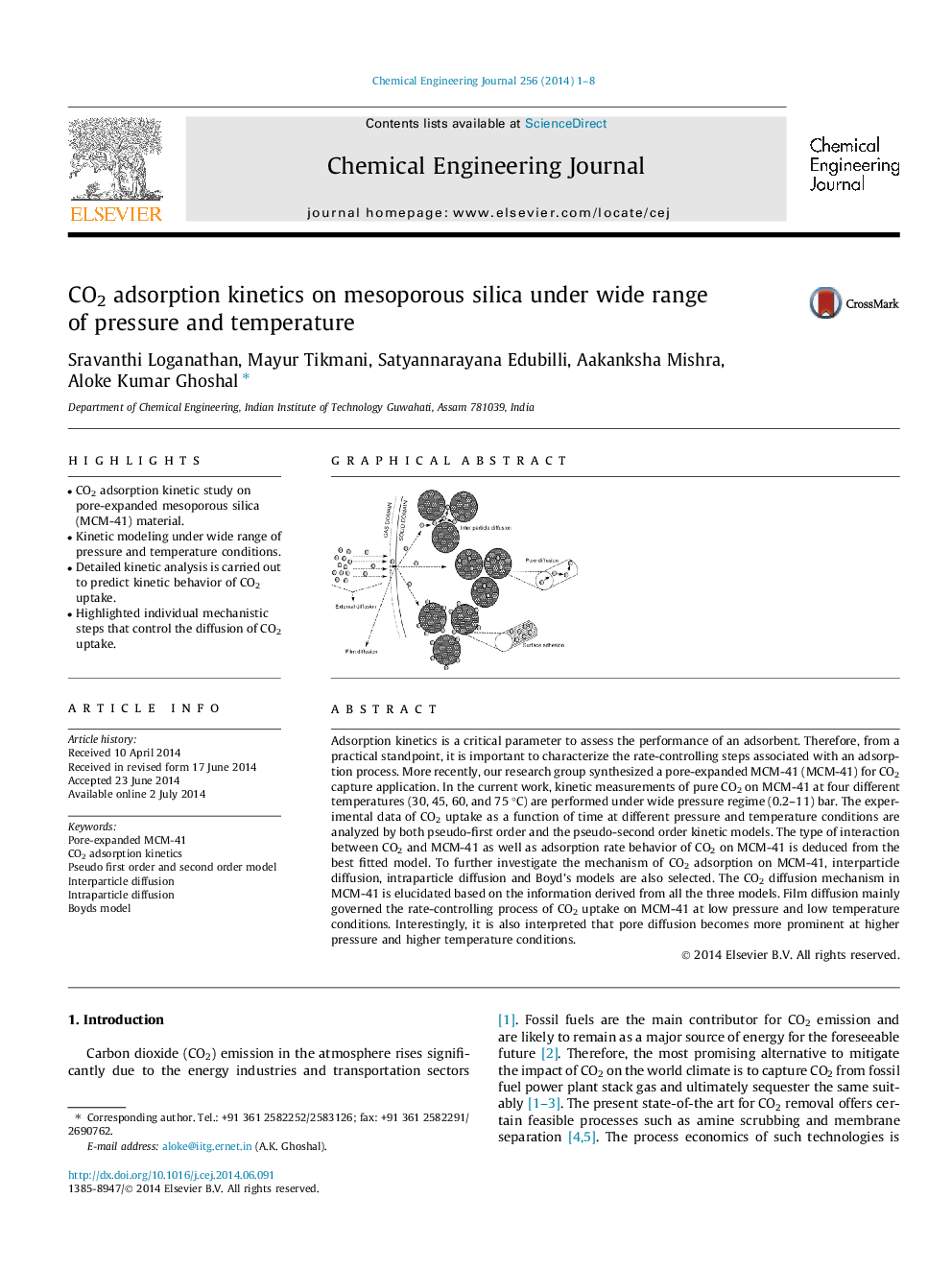
Adsorption kinetics is a critical parameter to assess the performance of an adsorbent. Therefore, from a practical standpoint, it is important to characterize the rate-controlling steps associated with an adsorption process. More recently, our research group synthesized a pore-expanded MCM-41 (MCM-41) for CO2 capture application. In the current work, kinetic measurements of pure CO2 on MCM-41 at four different temperatures (30, 45, 60, and 75 °C) are performed under wide pressure regime (0.2–11) bar. The experimental data of CO2 uptake as a function of time at different pressure and temperature conditions are analyzed by both pseudo-first order and the pseudo-second order kinetic models. The type of interaction between CO2 and MCM-41 as well as adsorption rate behavior of CO2 on MCM-41 is deduced from the best fitted model. To further investigate the mechanism of CO2 adsorption on MCM-41, interparticle diffusion, intraparticle diffusion and Boyd’s models are also selected. The CO2 diffusion mechanism in MCM-41 is elucidated based on the information derived from all the three models. Film diffusion mainly governed the rate-controlling process of CO2 uptake on MCM-41 at low pressure and low temperature conditions. Interestingly, it is also interpreted that pore diffusion becomes more prominent at higher pressure and higher temperature conditions.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 256, 15 November 2014, Pages 1–8