| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146994 | 456384 | 2014 | 8 صفحه PDF | دانلود رایگان |
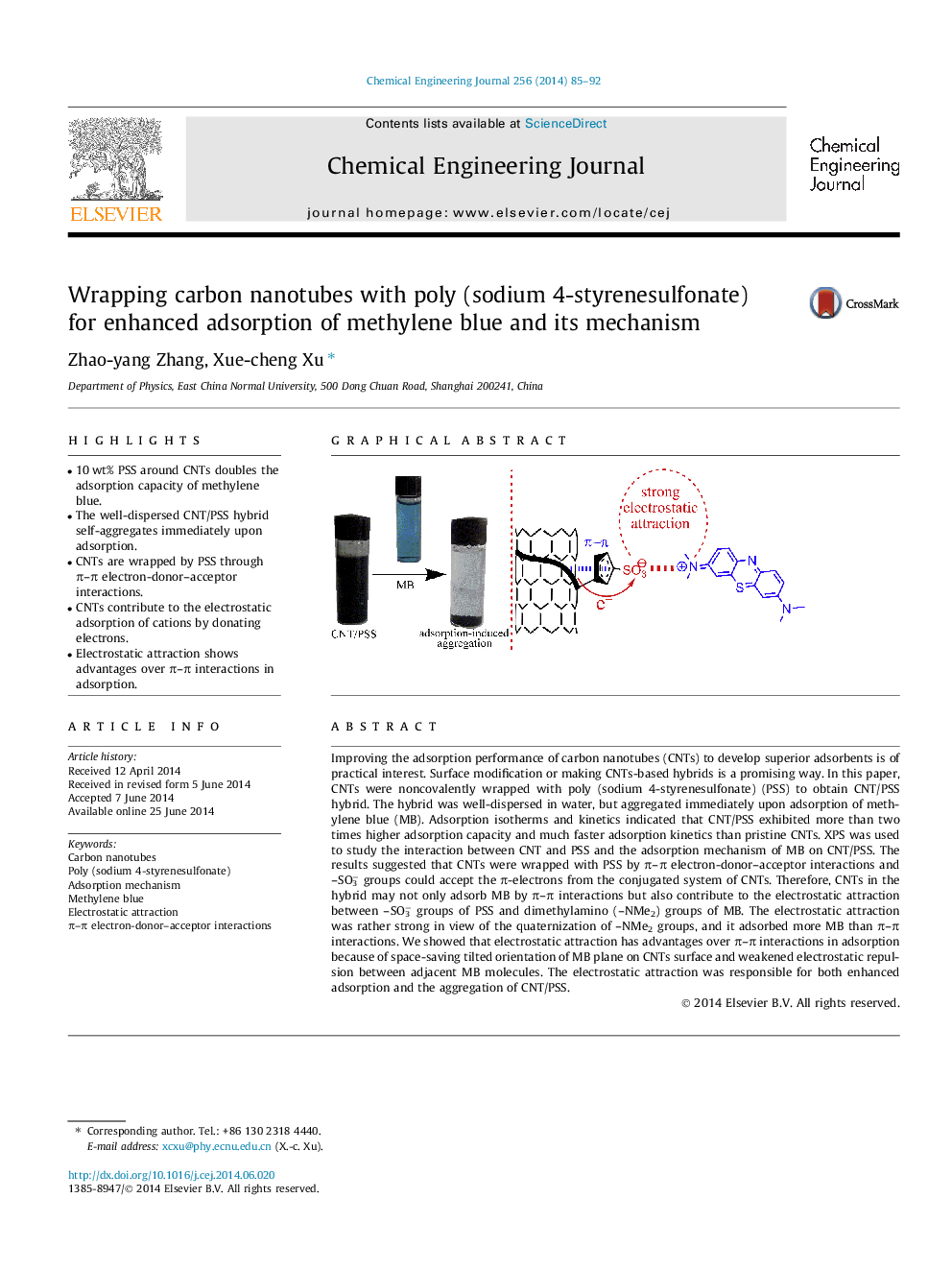
• 10 wt% PSS around CNTs doubles the adsorption capacity of methylene blue.
• The well-dispersed CNT/PSS hybrid self-aggregates immediately upon adsorption.
• CNTs are wrapped by PSS through π–π electron-donor–acceptor interactions.
• CNTs contribute to the electrostatic adsorption of cations by donating electrons.
• Electrostatic attraction shows advantages over π–π interactions in adsorption.
Improving the adsorption performance of carbon nanotubes (CNTs) to develop superior adsorbents is of practical interest. Surface modification or making CNTs-based hybrids is a promising way. In this paper, CNTs were noncovalently wrapped with poly (sodium 4-styrenesulfonate) (PSS) to obtain CNT/PSS hybrid. The hybrid was well-dispersed in water, but aggregated immediately upon adsorption of methylene blue (MB). Adsorption isotherms and kinetics indicated that CNT/PSS exhibited more than two times higher adsorption capacity and much faster adsorption kinetics than pristine CNTs. XPS was used to study the interaction between CNT and PSS and the adsorption mechanism of MB on CNT/PSS. The results suggested that CNTs were wrapped with PSS by π–π electron-donor–acceptor interactions and –SO3− groups could accept the π-electrons from the conjugated system of CNTs. Therefore, CNTs in the hybrid may not only adsorb MB by π–π interactions but also contribute to the electrostatic attraction between –SO3− groups of PSS and dimethylamino (–NMe2) groups of MB. The electrostatic attraction was rather strong in view of the quaternization of –NMe2 groups, and it adsorbed more MB than π–π interactions. We showed that electrostatic attraction has advantages over π–π interactions in adsorption because of space-saving tilted orientation of MB plane on CNTs surface and weakened electrostatic repulsion between adjacent MB molecules. The electrostatic attraction was responsible for both enhanced adsorption and the aggregation of CNT/PSS.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 256, 15 November 2014, Pages 85–92