| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147010 | 456384 | 2014 | 8 صفحه PDF | دانلود رایگان |
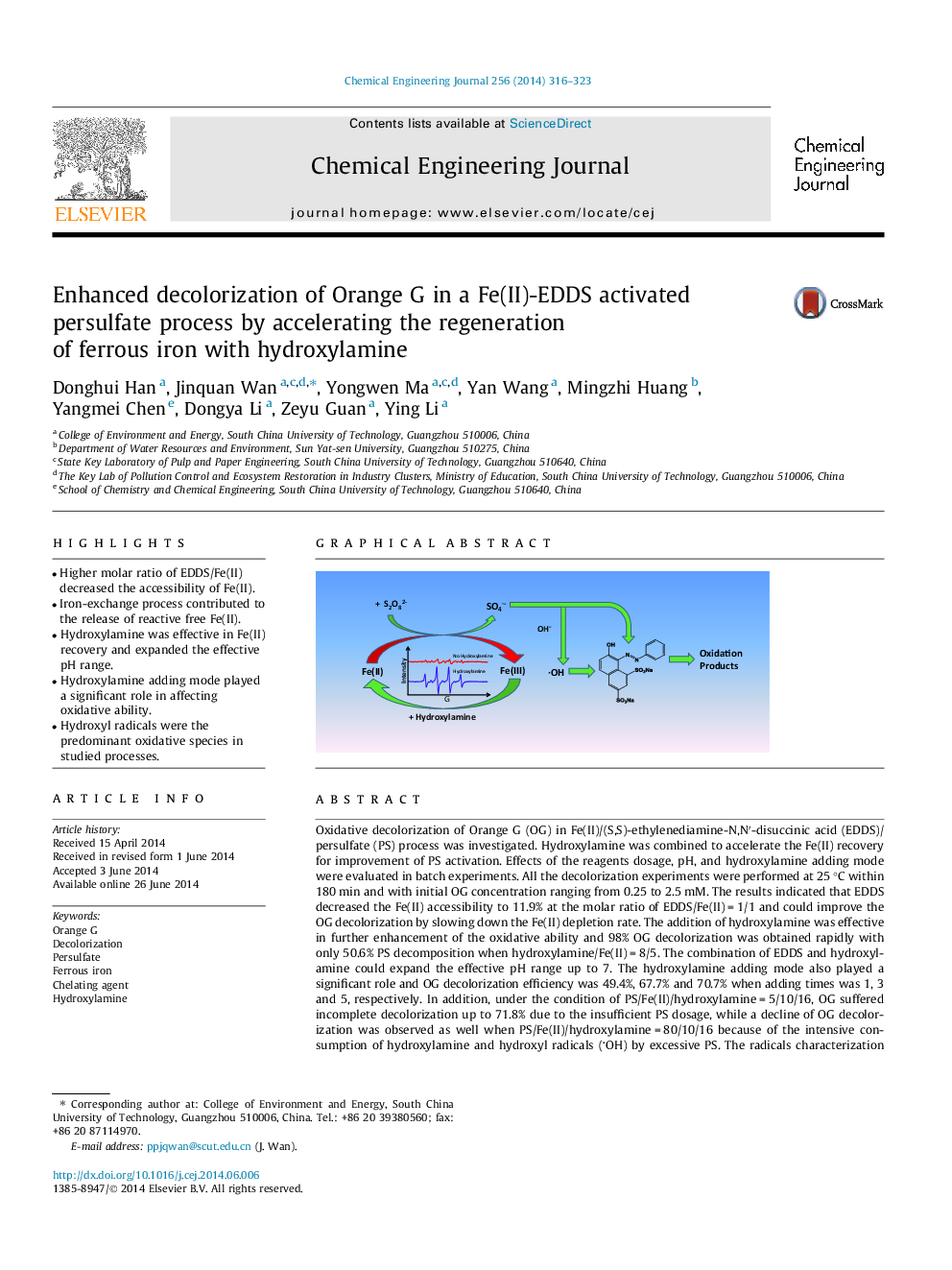
• Higher molar ratio of EDDS/Fe(II) decreased the accessibility of Fe(II).
• Iron-exchange process contributed to the release of reactive free Fe(II).
• Hydroxylamine was effective in Fe(II) recovery and expanded the effective pH range.
• Hydroxylamine adding mode played a significant role in affecting oxidative ability.
• Hydroxyl radicals were the predominant oxidative species in studied processes.
Oxidative decolorization of Orange G (OG) in Fe(II)/(S,S)-ethylenediamine-N,N′-disuccinic acid (EDDS)/persulfate (PS) process was investigated. Hydroxylamine was combined to accelerate the Fe(II) recovery for improvement of PS activation. Effects of the reagents dosage, pH, and hydroxylamine adding mode were evaluated in batch experiments. All the decolorization experiments were performed at 25 °C within 180 min and with initial OG concentration ranging from 0.25 to 2.5 mM. The results indicated that EDDS decreased the Fe(II) accessibility to 11.9% at the molar ratio of EDDS/Fe(II) = 1/1 and could improve the OG decolorization by slowing down the Fe(II) depletion rate. The addition of hydroxylamine was effective in further enhancement of the oxidative ability and 98% OG decolorization was obtained rapidly with only 50.6% PS decomposition when hydroxylamine/Fe(II) = 8/5. The combination of EDDS and hydroxylamine could expand the effective pH range up to 7. The hydroxylamine adding mode also played a significant role and OG decolorization efficiency was 49.4%, 67.7% and 70.7% when adding times was 1, 3 and 5, respectively. In addition, under the condition of PS/Fe(II)/hydroxylamine = 5/10/16, OG suffered incomplete decolorization up to 71.8% due to the insufficient PS dosage, while a decline of OG decolorization was observed as well when PS/Fe(II)/hydroxylamine = 80/10/16 because of the intensive consumption of hydroxylamine and hydroxyl radicals (OH) by excessive PS. The radicals characterization via electron spin resonance (ESR) technique was also conducted and the results indicated hydroxyl radicals were the predominant oxidative species in studied processes.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 256, 15 November 2014, Pages 316–323