| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147013 | 456384 | 2014 | 7 صفحه PDF | دانلود رایگان |
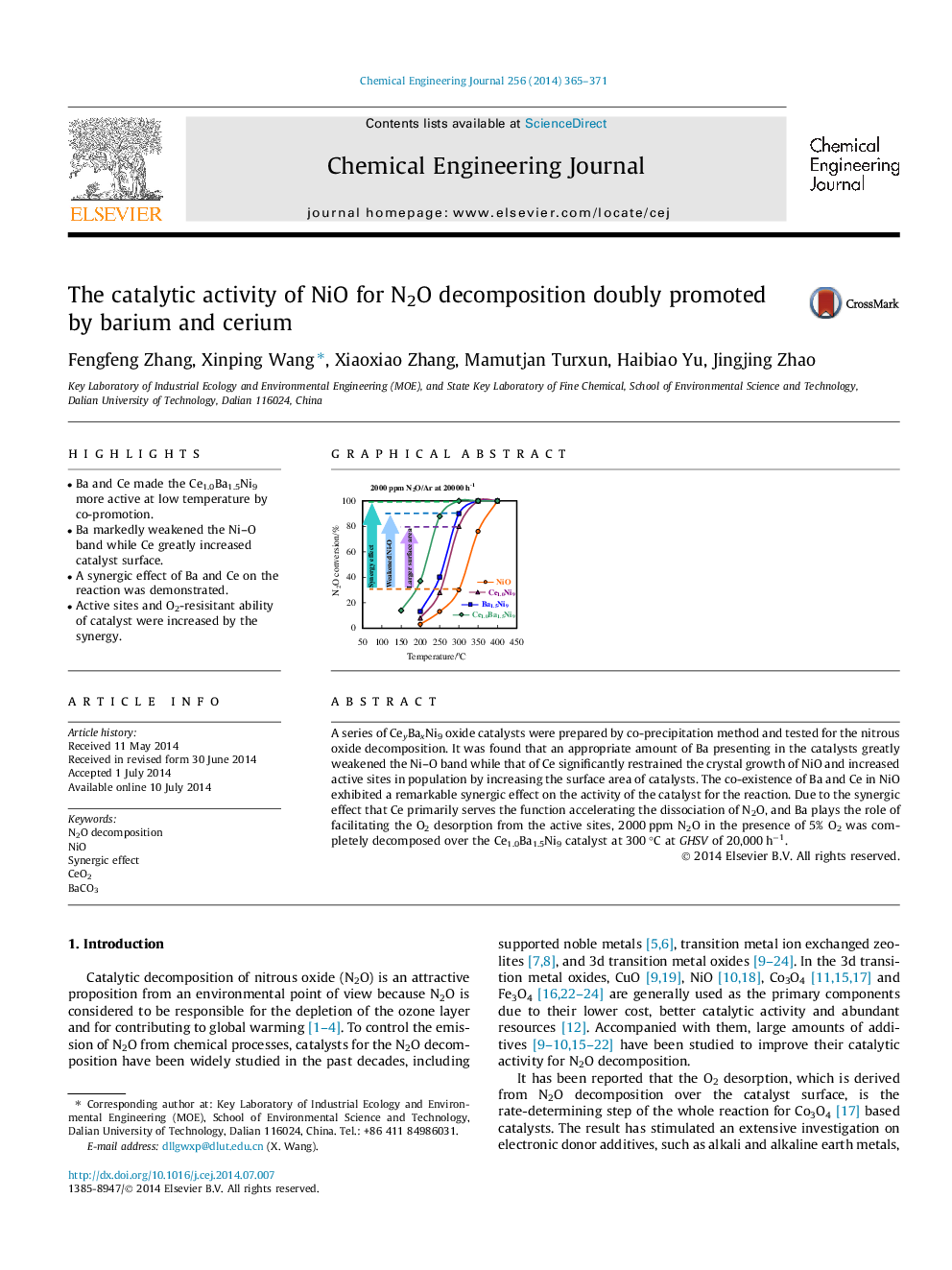
• Ba and Ce made the Ce1.0Ba1.5Ni9 more active at low temperature by co-promotion.
• Ba markedly weakened the Ni–O band while Ce greatly increased catalyst surface.
• A synergic effect of Ba and Ce on the reaction was demonstrated.
• Active sites and O2-resisitant ability of catalyst were increased by the synergy.
A series of CeyBaxNi9 oxide catalysts were prepared by co-precipitation method and tested for the nitrous oxide decomposition. It was found that an appropriate amount of Ba presenting in the catalysts greatly weakened the Ni–O band while that of Ce significantly restrained the crystal growth of NiO and increased active sites in population by increasing the surface area of catalysts. The co-existence of Ba and Ce in NiO exhibited a remarkable synergic effect on the activity of the catalyst for the reaction. Due to the synergic effect that Ce primarily serves the function accelerating the dissociation of N2O, and Ba plays the role of facilitating the O2 desorption from the active sites, 2000 ppm N2O in the presence of 5% O2 was completely decomposed over the Ce1.0Ba1.5Ni9 catalyst at 300 °C at GHSV of 20,000 h−1.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 256, 15 November 2014, Pages 365–371