| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147061 | 456385 | 2014 | 11 صفحه PDF | دانلود رایگان |
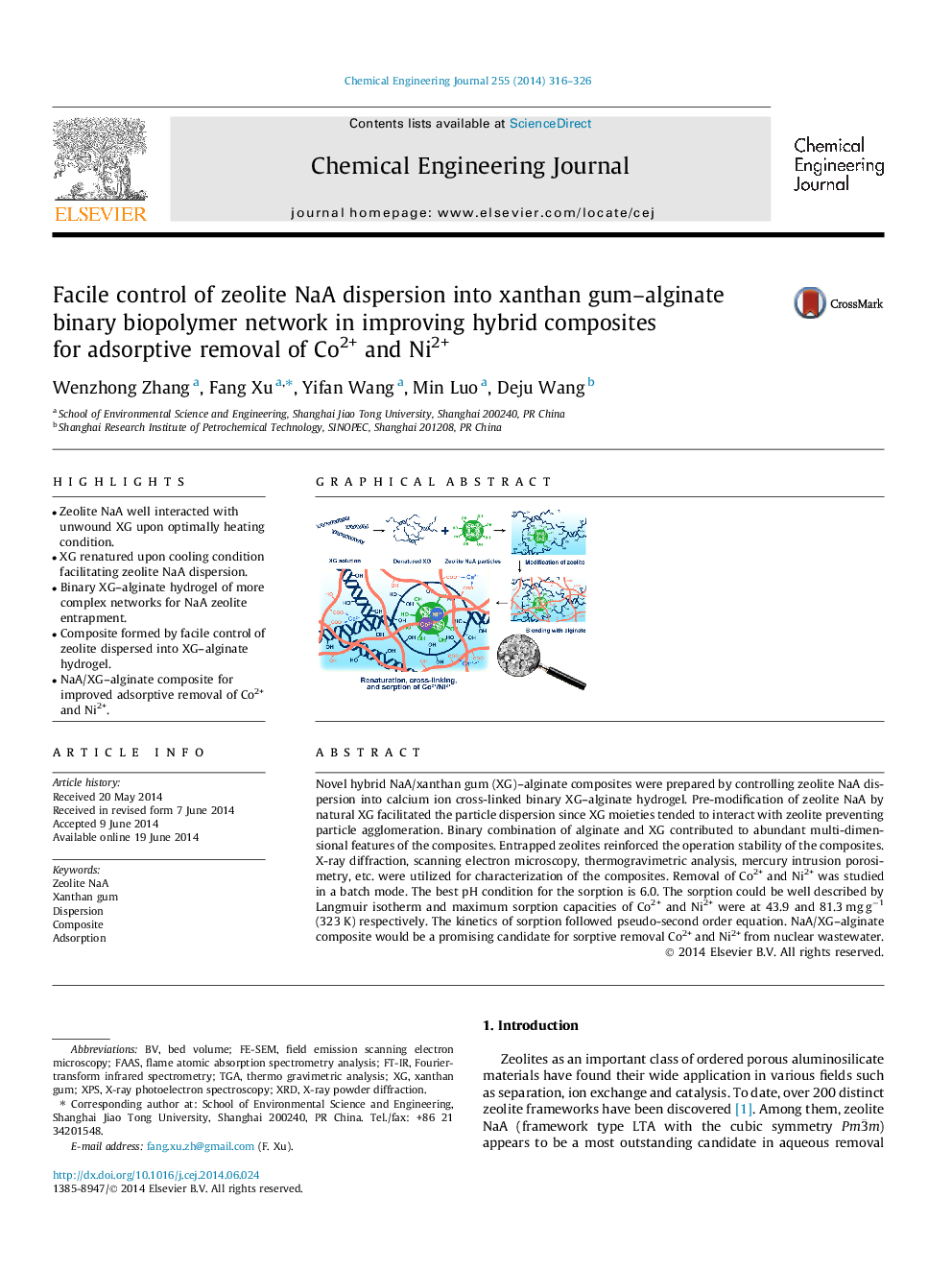
• Zeolite NaA well interacted with unwound XG upon optimally heating condition.
• XG renatured upon cooling condition facilitating zeolite NaA dispersion.
• Binary XG–alginate hydrogel of more complex networks for NaA zeolite entrapment.
• Composite formed by facile control of zeolite dispersed into XG–alginate hydrogel.
• NaA/XG–alginate composite for improved adsorptive removal of Co2+ and Ni2+.
Novel hybrid NaA/xanthan gum (XG)–alginate composites were prepared by controlling zeolite NaA dispersion into calcium ion cross-linked binary XG–alginate hydrogel. Pre-modification of zeolite NaA by natural XG facilitated the particle dispersion since XG moieties tended to interact with zeolite preventing particle agglomeration. Binary combination of alginate and XG contributed to abundant multi-dimensional features of the composites. Entrapped zeolites reinforced the operation stability of the composites. X-ray diffraction, scanning electron microscopy, thermogravimetric analysis, mercury intrusion porosimetry, etc. were utilized for characterization of the composites. Removal of Co2+ and Ni2+ was studied in a batch mode. The best pH condition for the sorption is 6.0. The sorption could be well described by Langmuir isotherm and maximum sorption capacities of Co2+ and Ni2+ were at 43.9 and 81.3 mg g−1 (323 K) respectively. The kinetics of sorption followed pseudo-second order equation. NaA/XG–alginate composite would be a promising candidate for sorptive removal Co2+ and Ni2+ from nuclear wastewater.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 255, 1 November 2014, Pages 316–326