| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147087 | 456385 | 2014 | 11 صفحه PDF | دانلود رایگان |
• Chemically cross-linked chitosan-poly(vinylamine) composites as beads.
• Increasing the sorption capacity for Cu2+ by in-situ chemical cross-linking.
• Sorption well described by Langmuir and Sips isotherms.
• Chemisorption supported by free energy of adsorption and pseudo-second-order kinetics.
• Chitosan-poly(vinylamine) beads with a remarkable level of reusability.
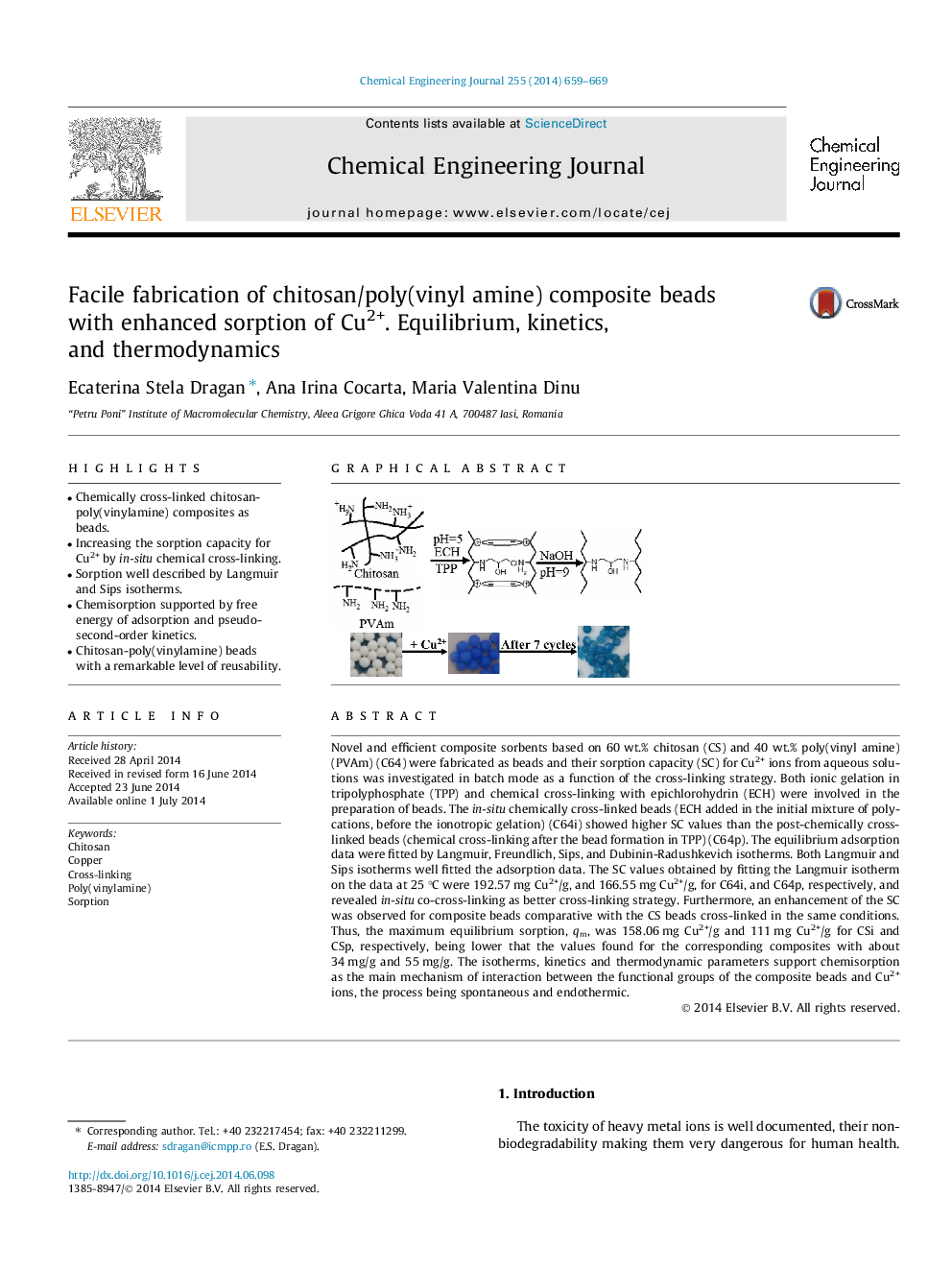
Novel and efficient composite sorbents based on 60 wt.% chitosan (CS) and 40 wt.% poly(vinyl amine) (PVAm) (C64) were fabricated as beads and their sorption capacity (SC) for Cu2+ ions from aqueous solutions was investigated in batch mode as a function of the cross-linking strategy. Both ionic gelation in tripolyphosphate (TPP) and chemical cross-linking with epichlorohydrin (ECH) were involved in the preparation of beads. The in-situ chemically cross-linked beads (ECH added in the initial mixture of polycations, before the ionotropic gelation) (C64i) showed higher SC values than the post-chemically cross-linked beads (chemical cross-linking after the bead formation in TPP) (C64p). The equilibrium adsorption data were fitted by Langmuir, Freundlich, Sips, and Dubinin-Radushkevich isotherms. Both Langmuir and Sips isotherms well fitted the adsorption data. The SC values obtained by fitting the Langmuir isotherm on the data at 25 °C were 192.57 mg Cu2+/g, and 166.55 mg Cu2+/g, for C64i, and C64p, respectively, and revealed in-situ co-cross-linking as better cross-linking strategy. Furthermore, an enhancement of the SC was observed for composite beads comparative with the CS beads cross-linked in the same conditions. Thus, the maximum equilibrium sorption, qm, was 158.06 mg Cu2+/g and 111 mg Cu2+/g for CSi and CSp, respectively, being lower that the values found for the corresponding composites with about 34 mg/g and 55 mg/g. The isotherms, kinetics and thermodynamic parameters support chemisorption as the main mechanism of interaction between the functional groups of the composite beads and Cu2+ ions, the process being spontaneous and endothermic.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 255, 1 November 2014, Pages 659–669