| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147155 | 456386 | 2014 | 14 صفحه PDF | دانلود رایگان |
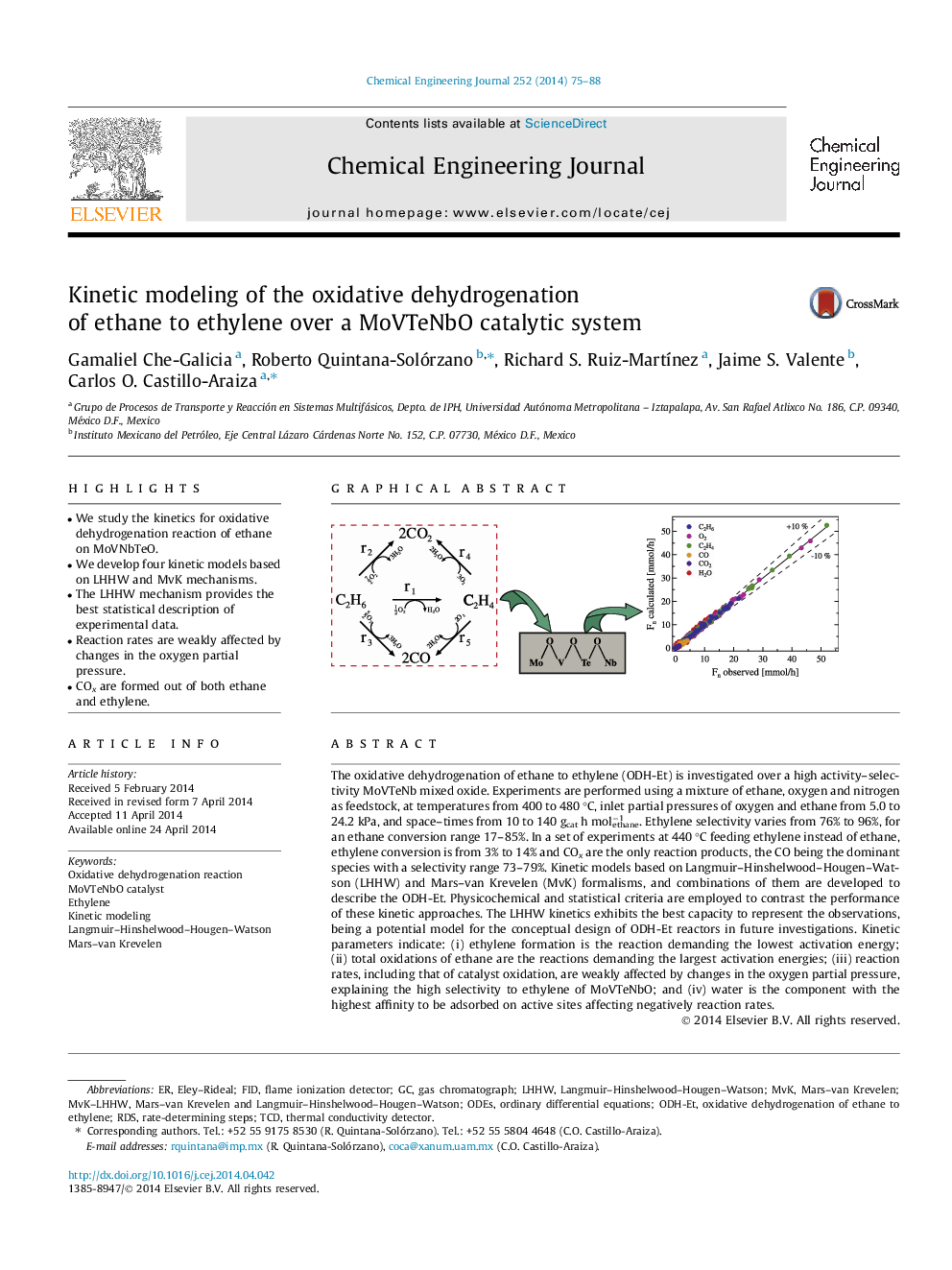
• We study the kinetics for oxidative dehydrogenation reaction of ethane on MoVNbTeO.
• We develop four kinetic models based on LHHW and MvK mechanisms.
• The LHHW mechanism provides the best statistical description of experimental data.
• Reaction rates are weakly affected by changes in the oxygen partial pressure.
• COx are formed out of both ethane and ethylene.
The oxidative dehydrogenation of ethane to ethylene (ODH-Et) is investigated over a high activity–selectivity MoVTeNb mixed oxide. Experiments are performed using a mixture of ethane, oxygen and nitrogen as feedstock, at temperatures from 400 to 480 °C, inlet partial pressures of oxygen and ethane from 5.0 to 24.2 kPa, and space–times from 10 to 140 gcat h molethane−1. Ethylene selectivity varies from 76% to 96%, for an ethane conversion range 17–85%. In a set of experiments at 440 °C feeding ethylene instead of ethane, ethylene conversion is from 3% to 14% and COx are the only reaction products, the CO being the dominant species with a selectivity range 73–79%. Kinetic models based on Langmuir–Hinshelwood–Hougen–Watson (LHHW) and Mars–van Krevelen (MvK) formalisms, and combinations of them are developed to describe the ODH-Et. Physicochemical and statistical criteria are employed to contrast the performance of these kinetic approaches. The LHHW kinetics exhibits the best capacity to represent the observations, being a potential model for the conceptual design of ODH-Et reactors in future investigations. Kinetic parameters indicate: (i) ethylene formation is the reaction demanding the lowest activation energy; (ii) total oxidations of ethane are the reactions demanding the largest activation energies; (iii) reaction rates, including that of catalyst oxidation, are weakly affected by changes in the oxygen partial pressure, explaining the high selectivity to ethylene of MoVTeNbO; and (iv) water is the component with the highest affinity to be adsorbed on active sites affecting negatively reaction rates.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 252, 15 September 2014, Pages 75–88