| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147252 | 456388 | 2014 | 10 صفحه PDF | دانلود رایگان |
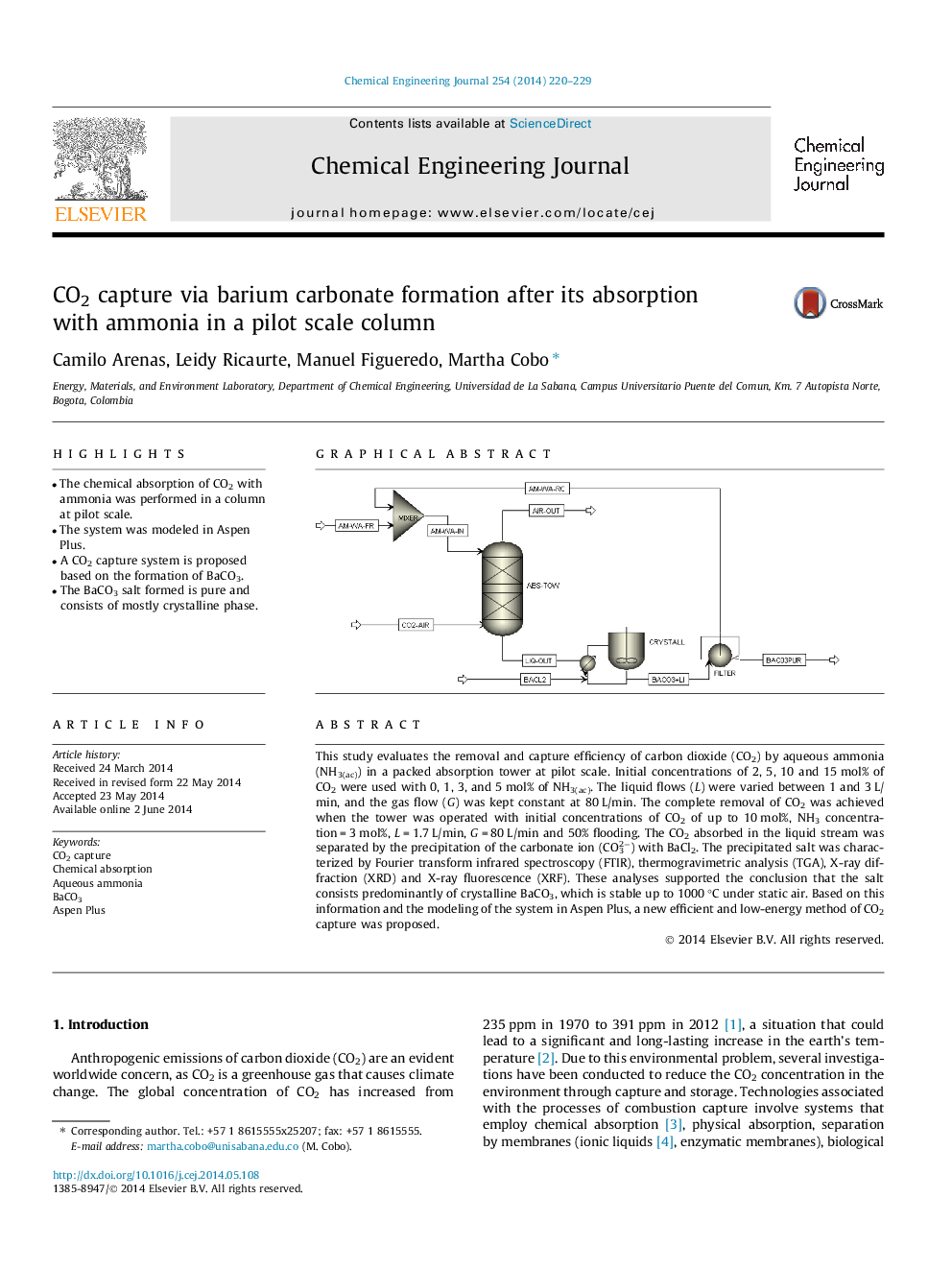
• The chemical absorption of CO2 with ammonia was performed in a column at pilot scale.
• The system was modeled in Aspen Plus.
• A CO2 capture system is proposed based on the formation of BaCO3.
• The BaCO3 salt formed is pure and consists of mostly crystalline phase.
This study evaluates the removal and capture efficiency of carbon dioxide (CO2) by aqueous ammonia (NH3(ac)) in a packed absorption tower at pilot scale. Initial concentrations of 2, 5, 10 and 15 mol% of CO2 were used with 0, 1, 3, and 5 mol% of NH3(ac). The liquid flows (L) were varied between 1 and 3 L/min, and the gas flow (G) was kept constant at 80 L/min. The complete removal of CO2 was achieved when the tower was operated with initial concentrations of CO2 of up to 10 mol%, NH3 concentration = 3 mol%, L = 1.7 L/min, G = 80 L/min and 50% flooding. The CO2 absorbed in the liquid stream was separated by the precipitation of the carbonate ion (CO32−) with BaCl2. The precipitated salt was characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), X-ray diffraction (XRD) and X-ray fluorescence (XRF). These analyses supported the conclusion that the salt consists predominantly of crystalline BaCO3, which is stable up to 1000 °C under static air. Based on this information and the modeling of the system in Aspen Plus, a new efficient and low-energy method of CO2 capture was proposed.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 254, 15 October 2014, Pages 220–229