| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147719 | 456398 | 2014 | 10 صفحه PDF | دانلود رایگان |
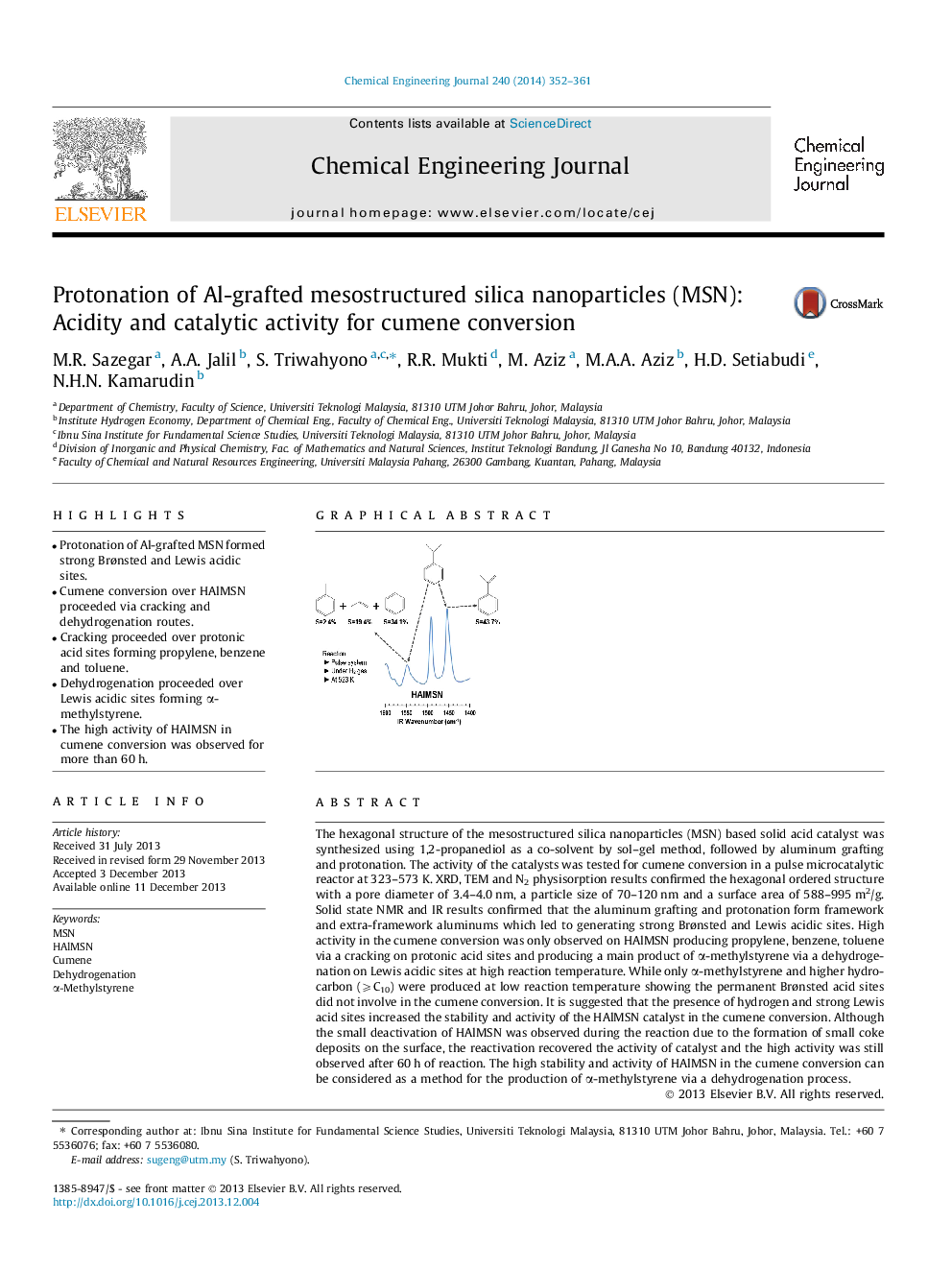
• Protonation of Al-grafted MSN formed strong Brønsted and Lewis acidic sites.
• Cumene conversion over HAlMSN proceeded via cracking and dehydrogenation routes.
• Cracking proceeded over protonic acid sites forming propylene, benzene and toluene.
• Dehydrogenation proceeded over Lewis acidic sites forming α-methylstyrene.
• The high activity of HAlMSN in cumene conversion was observed for more than 60 h.
The hexagonal structure of the mesostructured silica nanoparticles (MSN) based solid acid catalyst was synthesized using 1,2-propanediol as a co-solvent by sol–gel method, followed by aluminum grafting and protonation. The activity of the catalysts was tested for cumene conversion in a pulse microcatalytic reactor at 323–573 K. XRD, TEM and N2 physisorption results confirmed the hexagonal ordered structure with a pore diameter of 3.4–4.0 nm, a particle size of 70–120 nm and a surface area of 588–995 m2/g. Solid state NMR and IR results confirmed that the aluminum grafting and protonation form framework and extra-framework aluminums which led to generating strong Brønsted and Lewis acidic sites. High activity in the cumene conversion was only observed on HAlMSN producing propylene, benzene, toluene via a cracking on protonic acid sites and producing a main product of α-methylstyrene via a dehydrogenation on Lewis acidic sites at high reaction temperature. While only α-methylstyrene and higher hydrocarbon (⩾C10) were produced at low reaction temperature showing the permanent Brønsted acid sites did not involve in the cumene conversion. It is suggested that the presence of hydrogen and strong Lewis acid sites increased the stability and activity of the HAlMSN catalyst in the cumene conversion. Although the small deactivation of HAlMSN was observed during the reaction due to the formation of small coke deposits on the surface, the reactivation recovered the activity of catalyst and the high activity was still observed after 60 h of reaction. The high stability and activity of HAlMSN in the cumene conversion can be considered as a method for the production of α-methylstyrene via a dehydrogenation process.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 240, 15 March 2014, Pages 352–361