| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147878 | 456401 | 2014 | 8 صفحه PDF | دانلود رایگان |
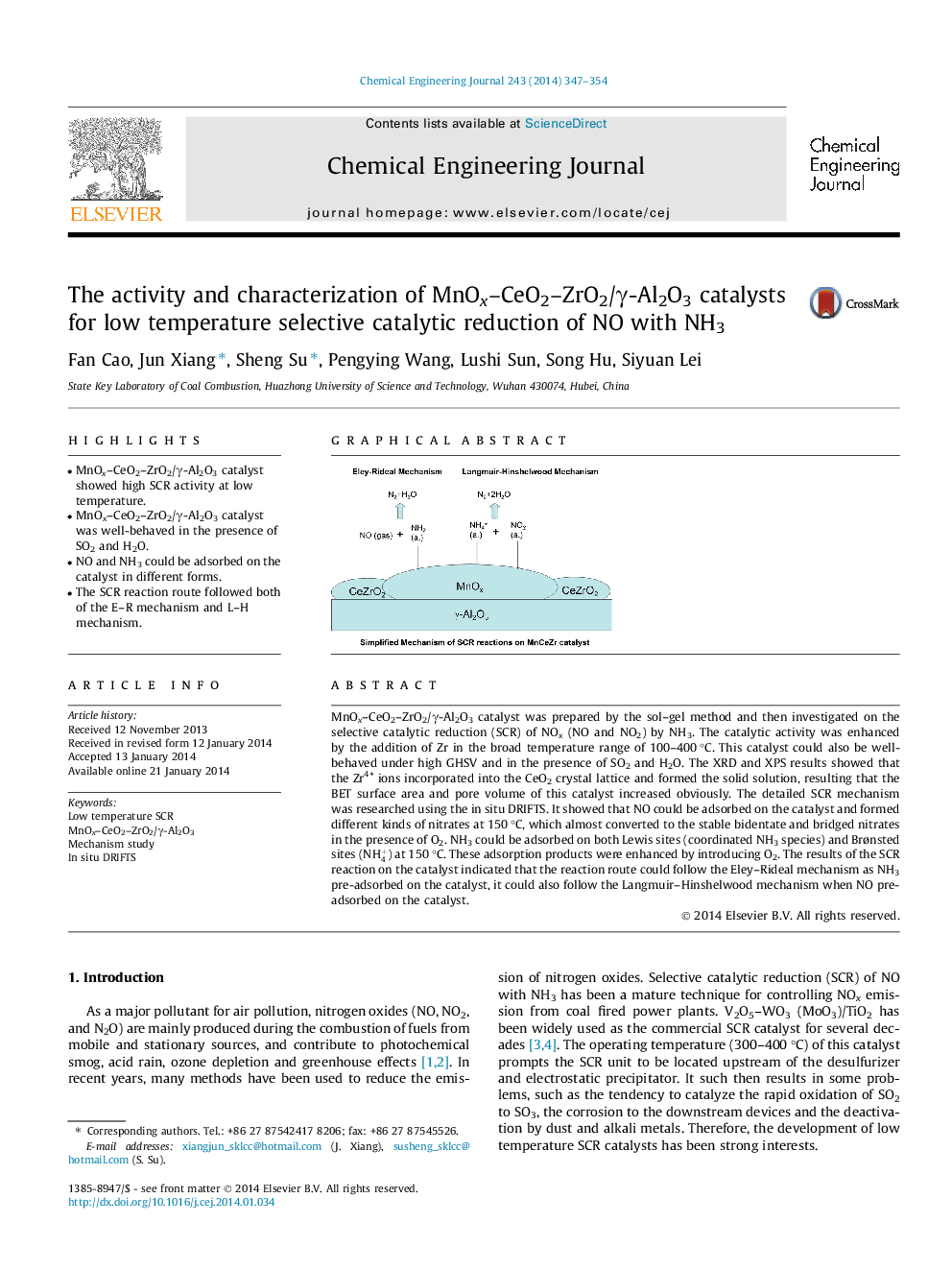
• MnOx–CeO2–ZrO2/γ-Al2O3 catalyst showed high SCR activity at low temperature.
• MnOx–CeO2–ZrO2/γ-Al2O3 catalyst was well-behaved in the presence of SO2 and H2O.
• NO and NH3 could be adsorbed on the catalyst in different forms.
• The SCR reaction route followed both of the E–R mechanism and L–H mechanism.
MnOx–CeO2–ZrO2/γ-Al2O3 catalyst was prepared by the sol–gel method and then investigated on the selective catalytic reduction (SCR) of NOx (NO and NO2) by NH3. The catalytic activity was enhanced by the addition of Zr in the broad temperature range of 100–400 °C. This catalyst could also be well-behaved under high GHSV and in the presence of SO2 and H2O. The XRD and XPS results showed that the Zr4+ ions incorporated into the CeO2 crystal lattice and formed the solid solution, resulting that the BET surface area and pore volume of this catalyst increased obviously. The detailed SCR mechanism was researched using the in situ DRIFTS. It showed that NO could be adsorbed on the catalyst and formed different kinds of nitrates at 150 °C, which almost converted to the stable bidentate and bridged nitrates in the presence of O2. NH3 could be adsorbed on both Lewis sites (coordinated NH3 species) and Brønsted sites (NH4+) at 150 °C. These adsorption products were enhanced by introducing O2. The results of the SCR reaction on the catalyst indicated that the reaction route could follow the Eley–Rideal mechanism as NH3 pre-adsorbed on the catalyst, it could also follow the Langmuir–Hinshelwood mechanism when NO pre-adsorbed on the catalyst.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 243, 1 May 2014, Pages 347–354