| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147949 | 456402 | 2014 | 9 صفحه PDF | دانلود رایگان |
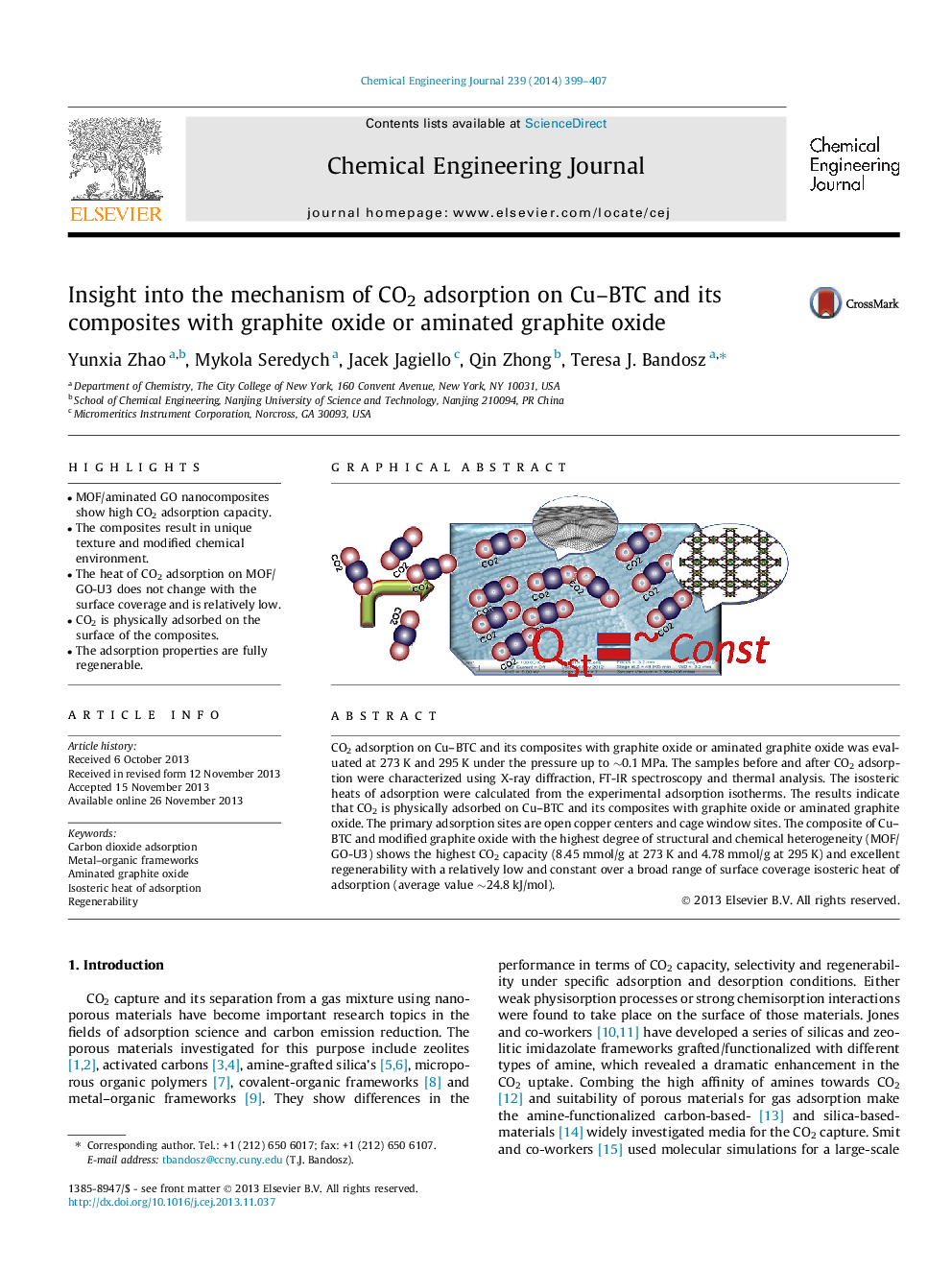
• MOF/aminated GO nanocomposites show high CO2 adsorption capacity.
• The composites result in unique texture and modified chemical environment.
• The heat of CO2 adsorption on MOF/GO-U3 does not change with the surface coverage and is relatively low.
• CO2 is physically adsorbed on the surface of the composites.
• The adsorption properties are fully regenerable.
CO2 adsorption on Cu–BTC and its composites with graphite oxide or aminated graphite oxide was evaluated at 273 K and 295 K under the pressure up to ∼0.1 MPa. The samples before and after CO2 adsorption were characterized using X-ray diffraction, FT-IR spectroscopy and thermal analysis. The isosteric heats of adsorption were calculated from the experimental adsorption isotherms. The results indicate that CO2 is physically adsorbed on Cu–BTC and its composites with graphite oxide or aminated graphite oxide. The primary adsorption sites are open copper centers and cage window sites. The composite of Cu–BTC and modified graphite oxide with the highest degree of structural and chemical heterogeneity (MOF/GO-U3) shows the highest CO2 capacity (8.45 mmol/g at 273 K and 4.78 mmol/g at 295 K) and excellent regenerability with a relatively low and constant over a broad range of surface coverage isosteric heat of adsorption (average value ∼24.8 kJ/mol).
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 239, 1 March 2014, Pages 399–407