| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 148407 | 456411 | 2013 | 6 صفحه PDF | دانلود رایگان |
• Catalytic oxidation of 1-butene to methylethylketone (MEK) is realized with S = 98%.
• The catalyst is an aqueous solution of Pd salt and heteropoly acid Н12P3Mo18V7O85.
• MEK is prepared at 60 °C, the catalyst is regenerated by oxygen of air at 170 °C.
• Kinetic equation obtained is used for calculation of the pilot tubular plug-flow reactor.
• Our homogeneous catalyst has increased thermal stability and is manufacturable.
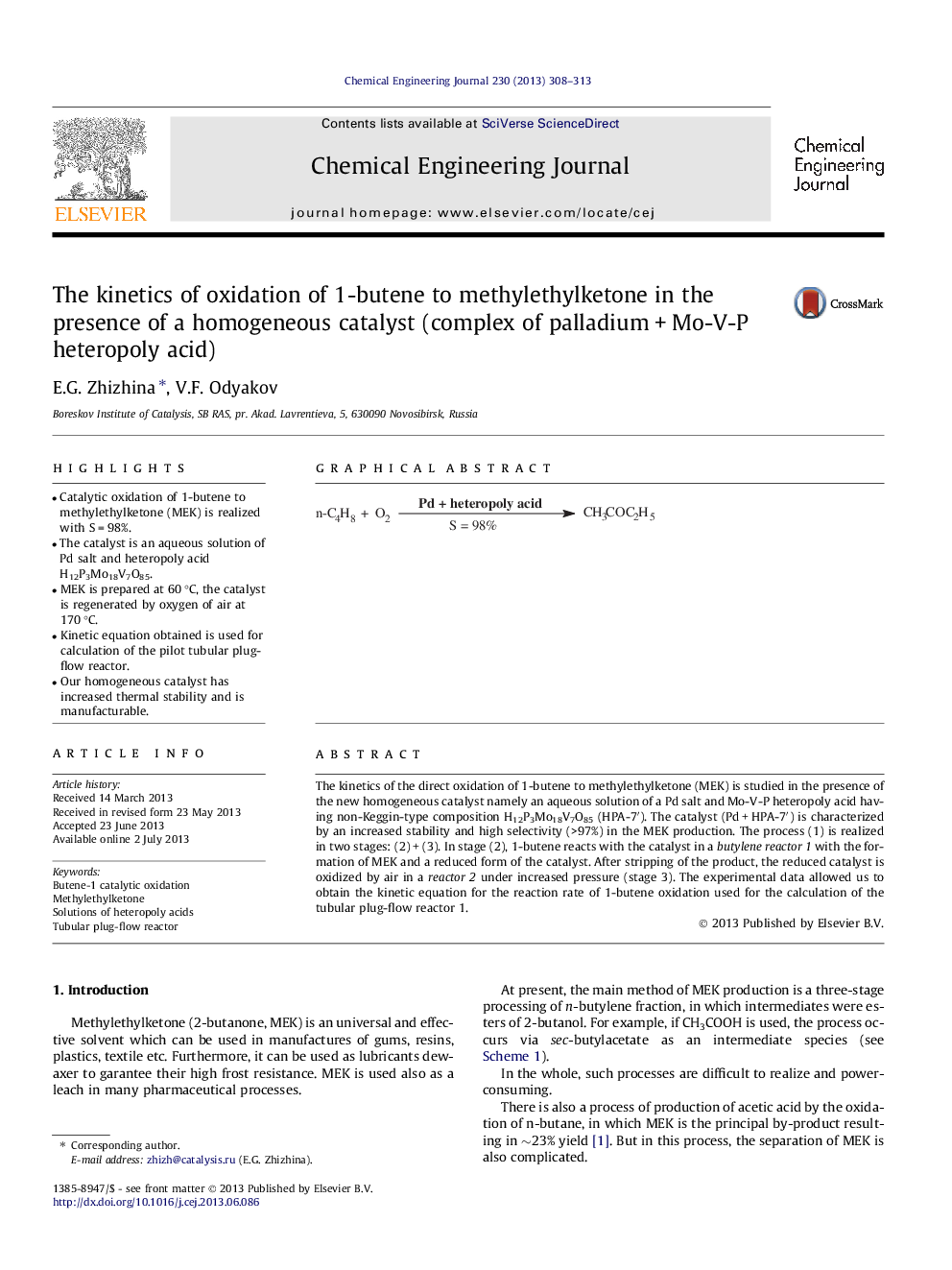
The kinetics of the direct oxidation of 1-butene to methylethylketone (MEK) is studied in the presence of the new homogeneous catalyst namely an aqueous solution of a Pd salt and Mo-V-P heteropoly acid having non-Keggin-type composition Н12P3Mo18V7O85 (HPA-7′). The catalyst (Pd + HPA-7′) is characterized by an increased stability and high selectivity (>97%) in the MEK production. The process (1) is realized in two stages: (2) + (3). In stage (2), 1-butene reacts with the catalyst in a butylene reactor 1 with the formation of MEK and a reduced form of the catalyst. After stripping of the product, the reduced catalyst is oxidized by air in a reactor 2 under increased pressure (stage 3). The experimental data allowed us to obtain the kinetic equation for the reaction rate of 1-butene oxidation used for the calculation of the tubular plug-flow reactor 1.
Graphical abstactFigure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 230, 15 August 2013, Pages 308–313