| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 148659 | 456421 | 2013 | 9 صفحه PDF | دانلود رایگان |
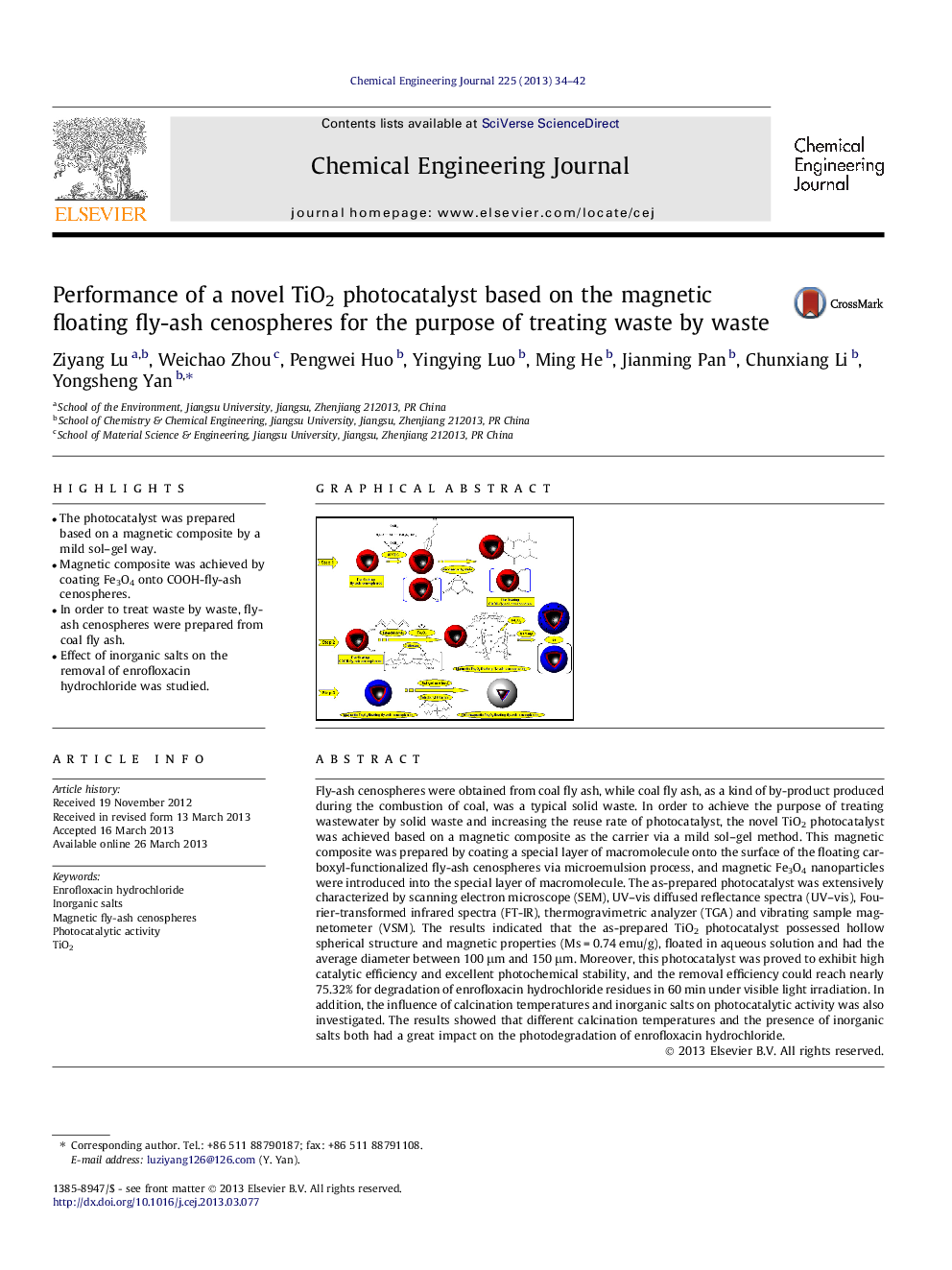
• The photocatalyst was prepared based on a magnetic composite by a mild sol–gel way.
• Magnetic composite was achieved by coating Fe3O4 onto COOH-fly-ash cenospheres.
• In order to treat waste by waste, fly-ash cenospheres were prepared from coal fly ash.
• Effect of inorganic salts on the removal of enrofloxacin hydrochloride was studied.
Fly-ash cenospheres were obtained from coal fly ash, while coal fly ash, as a kind of by-product produced during the combustion of coal, was a typical solid waste. In order to achieve the purpose of treating wastewater by solid waste and increasing the reuse rate of photocatalyst, the novel TiO2 photocatalyst was achieved based on a magnetic composite as the carrier via a mild sol–gel method. This magnetic composite was prepared by coating a special layer of macromolecule onto the surface of the floating carboxyl-functionalized fly-ash cenospheres via microemulsion process, and magnetic Fe3O4 nanoparticles were introduced into the special layer of macromolecule. The as-prepared photocatalyst was extensively characterized by scanning electron microscope (SEM), UV–vis diffused reflectance spectra (UV–vis), Fourier-transformed infrared spectra (FT-IR), thermogravimetric analyzer (TGA) and vibrating sample magnetometer (VSM). The results indicated that the as-prepared TiO2 photocatalyst possessed hollow spherical structure and magnetic properties (Ms = 0.74 emu/g), floated in aqueous solution and had the average diameter between 100 μm and 150 μm. Moreover, this photocatalyst was proved to exhibit high catalytic efficiency and excellent photochemical stability, and the removal efficiency could reach nearly 75.32% for degradation of enrofloxacin hydrochloride residues in 60 min under visible light irradiation. In addition, the influence of calcination temperatures and inorganic salts on photocatalytic activity was also investigated. The results showed that different calcination temperatures and the presence of inorganic salts both had a great impact on the photodegradation of enrofloxacin hydrochloride.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 225, 1 June 2013, Pages 34–42