| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 148709 | 456421 | 2013 | 11 صفحه PDF | دانلود رایگان |
• The anatase nanoparticles were successfully synthesized by sol–gel method.
• The kinetic data of lead, copper, and arsenic followed the pseudo-second-order model.
• The favorable Gibbs free energies of lead and copper were entropically controlled.
• The surface oxygen-containing functional groups were involved in the adsorption.
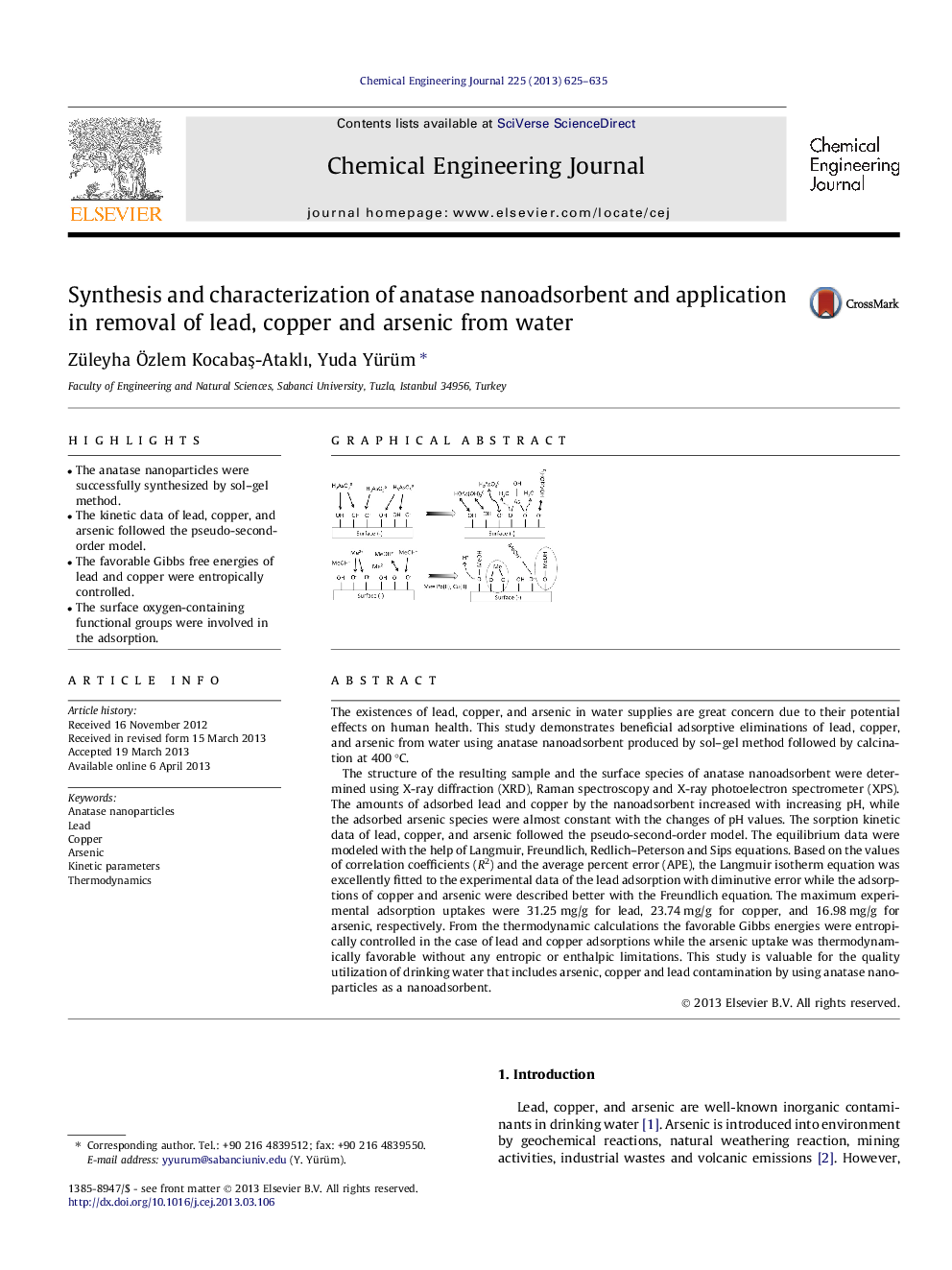
The existences of lead, copper, and arsenic in water supplies are great concern due to their potential effects on human health. This study demonstrates beneficial adsorptive eliminations of lead, copper, and arsenic from water using anatase nanoadsorbent produced by sol–gel method followed by calcination at 400 °C.The structure of the resulting sample and the surface species of anatase nanoadsorbent were determined using X-ray diffraction (XRD), Raman spectroscopy and X-ray photoelectron spectrometer (XPS). The amounts of adsorbed lead and copper by the nanoadsorbent increased with increasing pH, while the adsorbed arsenic species were almost constant with the changes of pH values. The sorption kinetic data of lead, copper, and arsenic followed the pseudo-second-order model. The equilibrium data were modeled with the help of Langmuir, Freundlich, Redlich–Peterson and Sips equations. Based on the values of correlation coefficients (R2) and the average percent error (APE), the Langmuir isotherm equation was excellently fitted to the experimental data of the lead adsorption with diminutive error while the adsorptions of copper and arsenic were described better with the Freundlich equation. The maximum experimental adsorption uptakes were 31.25 mg/g for lead, 23.74 mg/g for copper, and 16.98 mg/g for arsenic, respectively. From the thermodynamic calculations the favorable Gibbs energies were entropically controlled in the case of lead and copper adsorptions while the arsenic uptake was thermodynamically favorable without any entropic or enthalpic limitations. This study is valuable for the quality utilization of drinking water that includes arsenic, copper and lead contamination by using anatase nanoparticles as a nanoadsorbent.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 225, 1 June 2013, Pages 625–635