| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 149078 | 456426 | 2013 | 5 صفحه PDF | دانلود رایگان |
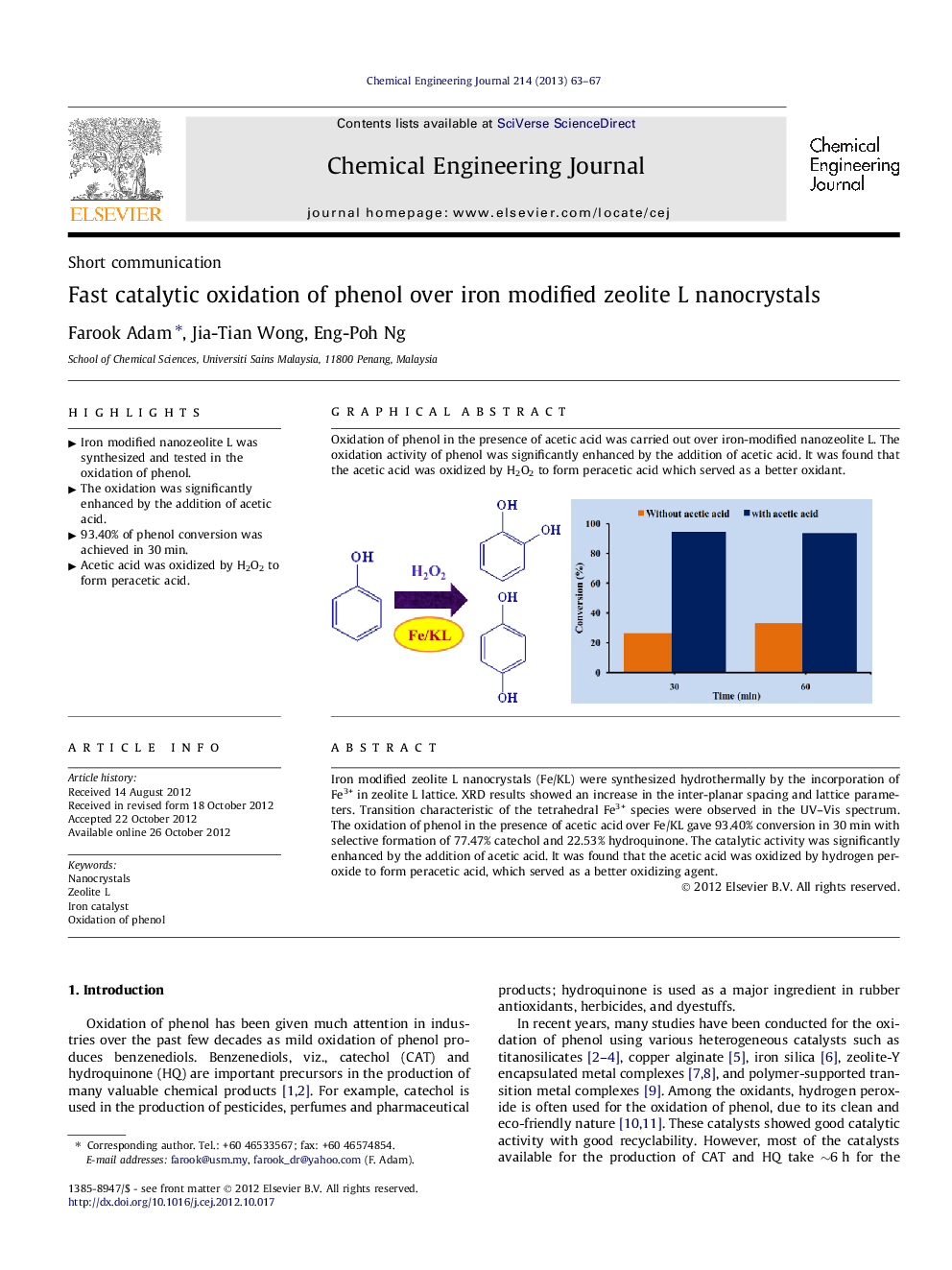
Iron modified zeolite L nanocrystals (Fe/KL) were synthesized hydrothermally by the incorporation of Fe3+ in zeolite L lattice. XRD results showed an increase in the inter-planar spacing and lattice parameters. Transition characteristic of the tetrahedral Fe3+ species were observed in the UV–Vis spectrum. The oxidation of phenol in the presence of acetic acid over Fe/KL gave 93.40% conversion in 30 min with selective formation of 77.47% catechol and 22.53% hydroquinone. The catalytic activity was significantly enhanced by the addition of acetic acid. It was found that the acetic acid was oxidized by hydrogen peroxide to form peracetic acid, which served as a better oxidizing agent.
Oxidation of phenol in the presence of acetic acid was carried out over iron-modified nanozeolite L. The oxidation activity of phenol was significantly enhanced by the addition of acetic acid. It was found that the acetic acid was oxidized by H2O2 to form peracetic acid which served as a better oxidant.Figure optionsDownload as PowerPoint slideHighlights
► Iron modified nanozeolite L was synthesized and tested in the oxidation of phenol.
► The oxidation was significantly enhanced by the addition of acetic acid.
► 93.40% of phenol conversion was achieved in 30 min.
► Acetic acid was oxidized by H2O2 to form peracetic acid.
Journal: Chemical Engineering Journal - Volume 214, 1 January 2013, Pages 63–67