| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1661130 | 1008418 | 2007 | 8 صفحه PDF | دانلود رایگان |
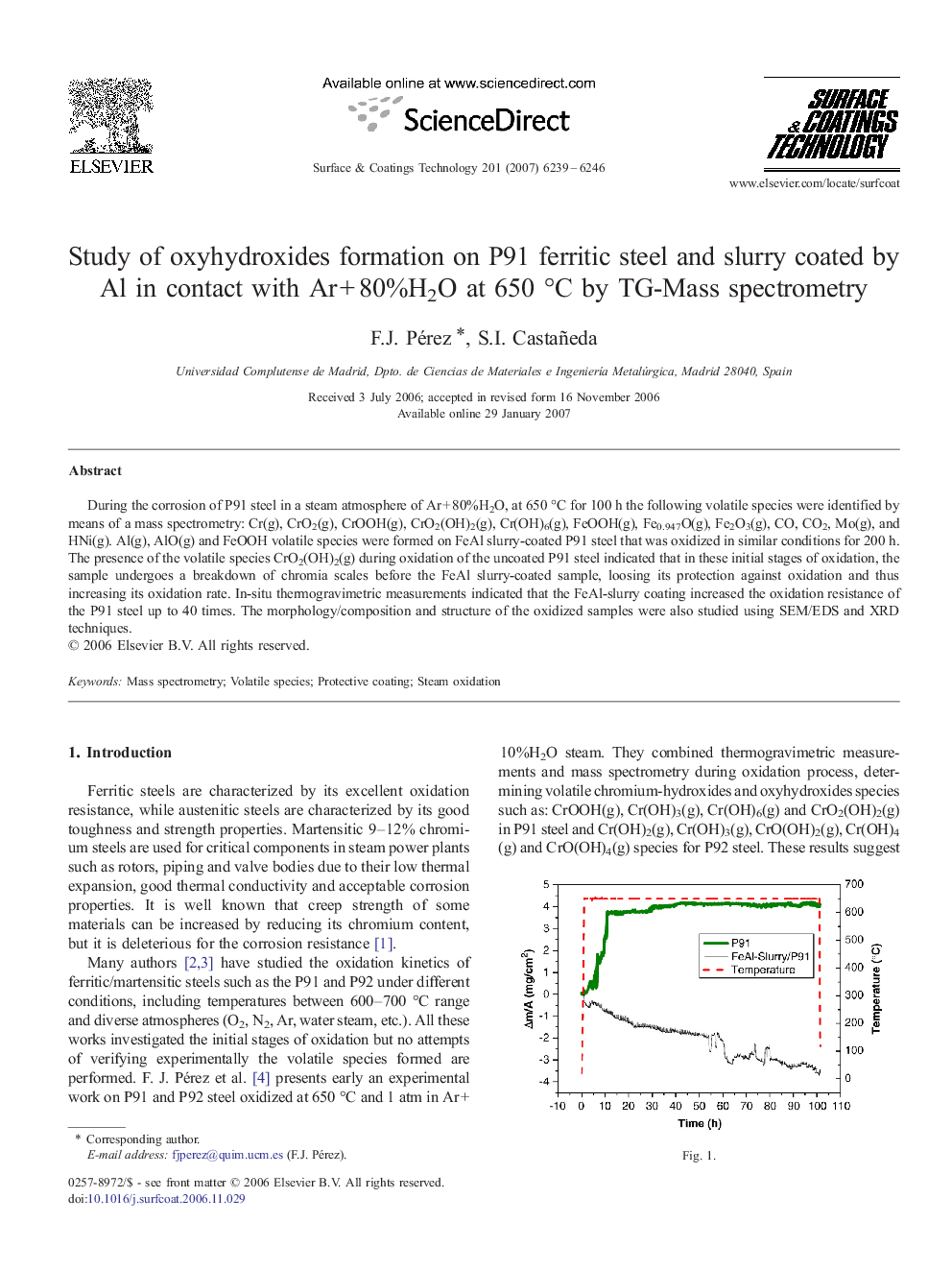
During the corrosion of P91 steel in a steam atmosphere of Ar + 80%H2O, at 650 °C for 100 h the following volatile species were identified by means of a mass spectrometry: Cr(g), CrO2(g), CrOOH(g), CrO2(OH)2(g), Cr(OH)6(g), FeOOH(g), Fe0.947O(g), Fe2O3(g), CO, CO2, Mo(g), and HNi(g). Al(g), AlO(g) and FeOOH volatile species were formed on FeAl slurry-coated P91 steel that was oxidized in similar conditions for 200 h. The presence of the volatile species CrO2(OH)2(g) during oxidation of the uncoated P91 steel indicated that in these initial stages of oxidation, the sample undergoes a breakdown of chromia scales before the FeAl slurry-coated sample, loosing its protection against oxidation and thus increasing its oxidation rate. In-situ thermogravimetric measurements indicated that the FeAl-slurry coating increased the oxidation resistance of the P91 steel up to 40 times. The morphology/composition and structure of the oxidized samples were also studied using SEM/EDS and XRD techniques.
Journal: Surface and Coatings Technology - Volume 201, Issue 14, 2 April 2007, Pages 6239–6246