| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 205276 | 461102 | 2016 | 9 صفحه PDF | دانلود رایگان |
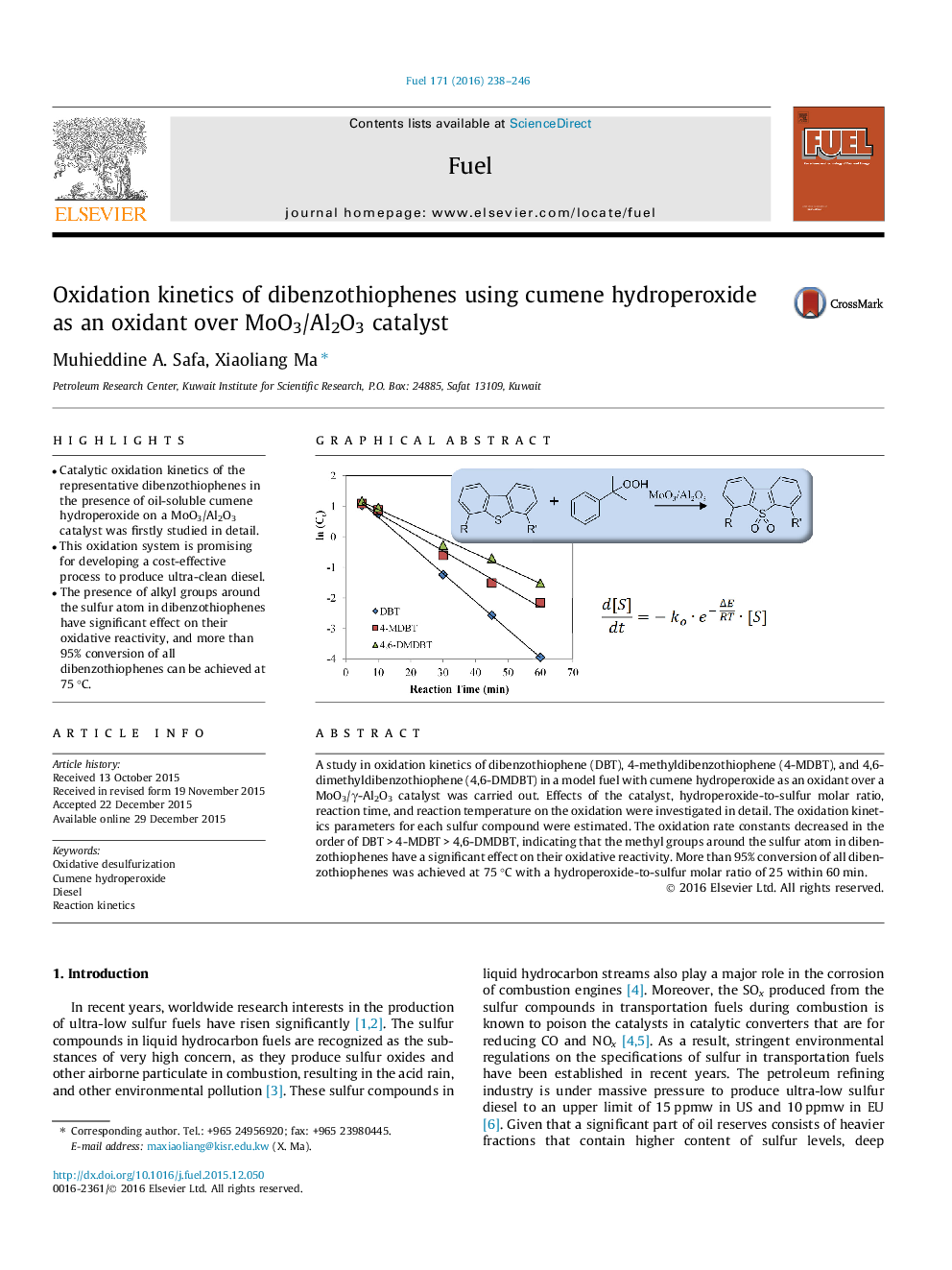
• Catalytic oxidation kinetics of the representative dibenzothiophenes in the presence of oil-soluble cumene hydroperoxide on a MoO3/Al2O3 catalyst was firstly studied in detail.
• This oxidation system is promising for developing a cost-effective process to produce ultra-clean diesel.
• The presence of alkyl groups around the sulfur atom in dibenzothiophenes have significant effect on their oxidative reactivity, and more than 95% conversion of all dibenzothiophenes can be achieved at 75 °C.
A study in oxidation kinetics of dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT) in a model fuel with cumene hydroperoxide as an oxidant over a MoO3/γ-Al2O3 catalyst was carried out. Effects of the catalyst, hydroperoxide-to-sulfur molar ratio, reaction time, and reaction temperature on the oxidation were investigated in detail. The oxidation kinetics parameters for each sulfur compound were estimated. The oxidation rate constants decreased in the order of DBT > 4-MDBT > 4,6-DMDBT, indicating that the methyl groups around the sulfur atom in dibenzothiophenes have a significant effect on their oxidative reactivity. More than 95% conversion of all dibenzothiophenes was achieved at 75 °C with a hydroperoxide-to-sulfur molar ratio of 25 within 60 min.
Figure optionsDownload as PowerPoint slide
Journal: Fuel - Volume 171, 1 May 2016, Pages 238–246