| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 216562 | 1426279 | 2011 | 6 صفحه PDF | دانلود رایگان |
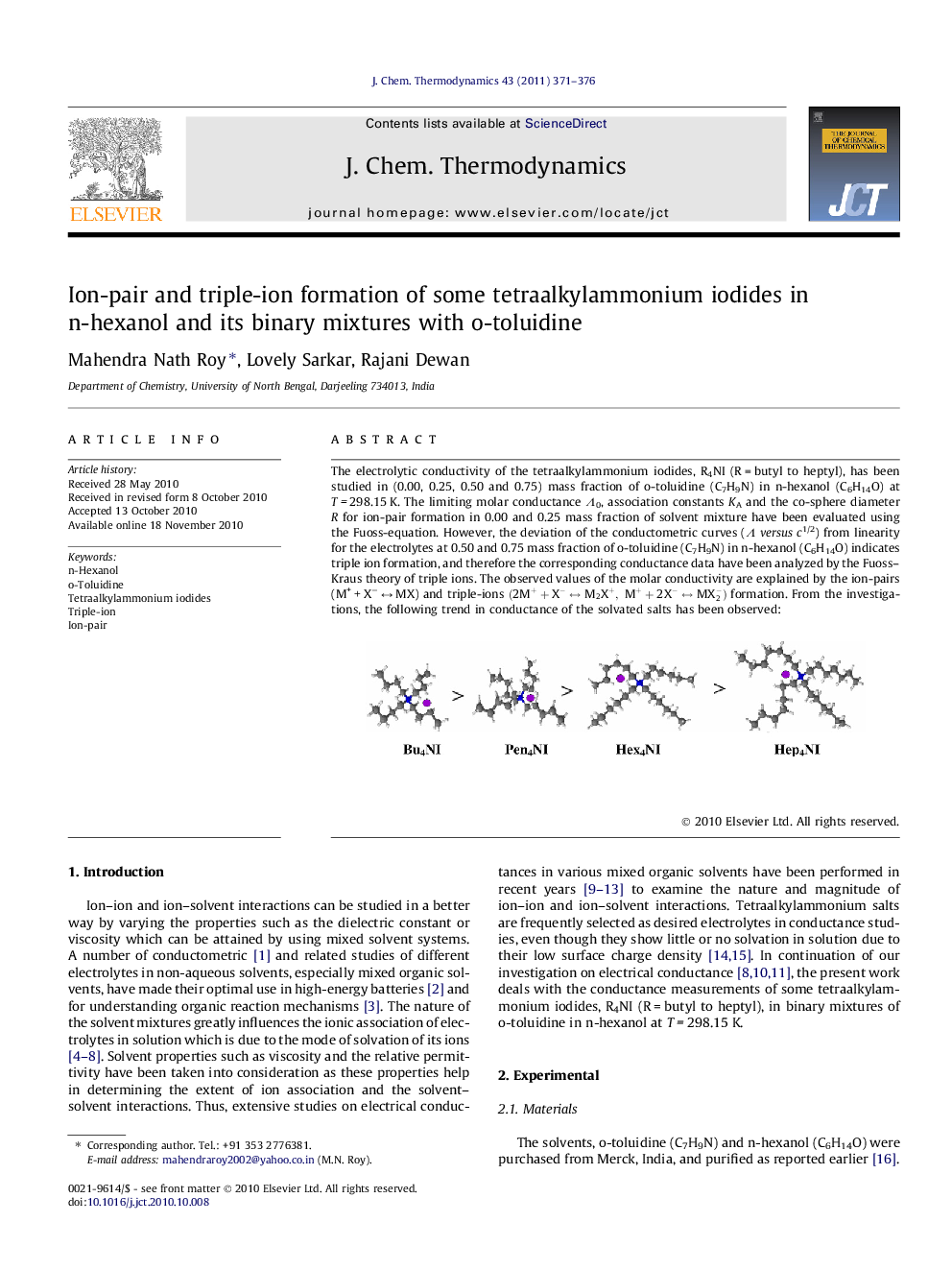
The electrolytic conductivity of the tetraalkylammonium iodides, R4NI (R = butyl to heptyl), has been studied in (0.00, 0.25, 0.50 and 0.75) mass fraction of o-toluidine (C7H9N) in n-hexanol (C6H14O) at T = 298.15 K. The limiting molar conductance Λ0, association constants KA and the co-sphere diameter R for ion-pair formation in 0.00 and 0.25 mass fraction of solvent mixture have been evaluated using the Fuoss-equation. However, the deviation of the conductometric curves (Λversusc1/2) from linearity for the electrolytes at 0.50 and 0.75 mass fraction of o-toluidine (C7H9N) in n-hexanol (C6H14O) indicates triple ion formation, and therefore the corresponding conductance data have been analyzed by the Fuoss–Kraus theory of triple ions. The observed values of the molar conductivity are explained by the ion-pairs (M+ + X− ↔ MX) and triple-ions (2M++X-↔M2X+,M++2X-↔MX2-) formation. From the investigations, the following trend in conductance of the solvated salts has been observed:Figure optionsDownload as PowerPoint slide
Journal: The Journal of Chemical Thermodynamics - Volume 43, Issue 3, March 2011, Pages 371–376